Formation damage reduces permeability of reservoirs thereby reducing well productivity. One of the methods that is used to remove formation damage is acidization. Oilfield scales are basically mineral scales that are formed during petroleum production and have the tendency to cause wellbore damage. Formation of oilfield scales result predominantly from over saturation of dissolved minerals in oil and gas produced water. In this study, scale samples have been analysed and identified using x-ray diffractometry. X-Ray diffractometry was used to identify minerals present in the wellbore scales. The X-Ray diffractometry results confirmed the presence of seventeen different minerals in the scales and these have been classified as carbonates, silicates and phosphates. Matrix acidizing fluids have also been designed and formulated to test the capability to dissolving the scales. 10% and 15% HCl (regular acids) as well as xylene and diesel were used on the scales to ascertain their dissolution capabilities. None of these three fluids was able to completely dissolve the sample scales because the mineralogical compositions revealed by XRD were mainly the HCl insoluble minerals such as Petalite, Xenotite, etc. Only 10% mineral composition of the scales identified were HCl soluble. The results of this study prove that, X-Ray Diffractometry must precede any action taken to remove/treat oilfield scales, especially when considering chemical dissolution. This will prevent wastage and save money and time.
Keywords: Oilfields scales, Acidizing fluids, Wellbore damage, Mineral scales, Dissolution
Oilfield scales (principally barium sulphate, calcium carbonate) are the mineral precipitates which form in the formation, production tubing and surface equipment due to downhole fluid chemistry and environmental conditions. Scales deposition is a great concern to the oil industry, since it can present significant problems in terms of productivity and safety. Particular reference is made to a case-study in the North Sea oilfield where barium sulphate scale formation occurs due to mixing of incompatible injected seawater and formation brine.1 Oilfield scales lead to formation damage as well as wellbore damage.
Scales are deposits/coatings which form on surfaces of metals, rocks and other materials. Scale formation results from precipitation from chemical reaction with surfaces, pressure and temperature changes, or changes in the composition of solutions. Examples of scales are calcium sulfate, iron oxides, barium sulphate, calcium carbonate, strontium sulphate. Also, iron sulfide, iron carbonate, phosphates, silicates and oxides, as well as number of compounds insoluble or marginally soluble in water.
Formation damage is any process that leads to decrease in the natural productivity of an oil or gas producing formation, or reduction in injection capacity of a water or gas injection well. Civan2 defines formation damage as permeability and porosity variation by the following processes: fines migration and deposition, mud filtrate and fines invasion, rock compression, scales and acidizing. Formation Damage is also described as any mechanical, chemical, biological or thermal process in a reservoir that causes a decrease in permeability and porosity.3
Formation damage may occur through drilling operations, the effect of long-term production, fines migration, completion and stimulation fluids.4 Recovery from these damages can be improved through productivity enhancement techniques which remove or bypass the damage near the wellbore region. Damages due to plugging of perforations and rock matrix through debris from well and its operations can restrict the flow of reservoir fluids into the wellbore. The deposition of materials in the formation pore throats could prevent the normal natural flow of hydrocarbon towards the well bore. A disadvantage of scales deposition is that, it reduces the near wellbore permeability in the reservoir; resulting in high skin and lower inflow.5
Matrix acidizing in sandstone formation has been one of the effective means in well stimulation, and the technology has been applied in the field with low success, especially compared with hydraulic fracturing.6 Sandstone matrix acidizing has been in use for improving oil and gas production by removing damages thereby increasing permeability of near wellbore area. The first matrix acidizing jobs were successful in enhancing oil production in carbonates formation. However, recent attention to matrix acidizing has been directed to sandstones and the utilisation of various hydrofluoric acid (HF) systems.7
Hydrochloric acid has remained the principal acid treating agent for most wells since the first commercial use in the 1930s due to its effectiveness and moderately low cost. Other acids including; formic, acetic, hydrofluoric have been also employed for other applications such as deep hot wells and normal well treatments in sandstone reservoirs.8
The study covers the design of various acidizing systems comprising of regular acids, mud acids, non-retarded, retarded acids as well as acid diverter systems. The acids used in this research are all inorganic acids.
Reservoir scales are collected and identified through common dissolution test with some selected acidizing fluid systems whiles a more comprehensive X-Ray Diffraction method is used for identifying mineralogy of scales.
X-ray diffraction (XRD) is the primary, non-destructive tool for identifying and quantifying the mineralogy of crystalline compounds in rocks, soils and particulates. Every mineral or compound has a characteristic X-ray diffraction pattern whose 'fingerprint' can be matched against a database of over 250 000 recorded phases. Modern computer-controlled diffraction systems can interpret the diffraction traces produced by individual constituents and highly complex mixtures. XRD is an essential technique for identifying and characterising the nature of clay minerals, providing information which cannot be determined by any other method. X-ray Diffraction is a technique used by mineralogists to examine the physical and chemical composition of solids. Data is represented in a collection of single-phase X-Ray powder diffraction patterns for three intense “D” values in tables form of interplanar spacings (D), relative intensities (I/Io), and name of the mineral as shown in Bragg’s equation (2.9):
nλ=2d sinθ Equation 1Where;
λ= wave length
d= inter atomic spacing
Ө= angle between the incident rays and the surface of the crystal
n= order of reflection
During the process, the technique takes a material sample and places a powdered sample in a holder, the sample is then illuminated with X-Rays of fixed wave-length and a goniometer is used to record the intensity of the reflected radiation. This data further goes through analysis for the reflection angle to calculate the inter-atomic spacing (D value in Angstrom units - 10-8 cm). The intensity (I) is measured to discriminate (using I ratios) the various D spacings and results are to identify possible matches (Anon, 2016).9,10
Experimental procedures
The following are the key tests performed in this research. The tests include scale analysis through X-Ray Diffractometry and scale dissolution with acidizing fluids.
X-Ray Diffractometry (XRD) of wellbore scales
Scale samples were obtained from wellbores within the Niger Delta region for mineralogical analysis using XRD (X Pert PRO). The analysis reveals the minerals making up the scales, for scale type identification and this makes it easy to prescribe the right kinds of treatment fluid which is able to dissolve off the scales in the wellbore. The XRD results also reveal the percentage weight of each of the minerals present in the scale sample. The minerals with their individual percentages identified by this process enable the right treatment fluids to be designed to remove/treat them. It is possible for scales to compose of both acid soluble and acid insoluble minerals and thus other techniques have to be found to treat/remove such scales.
In a XRD test, incident wave is focused unto a substance and a detector is normally moved to record the directions and intensities of the diffracted waves.11 “Coherent scattering” conserves the exactness of wave periodicity. Positive or negative interference occurs through various directions as dispersed waves are discharged by atoms of diverse types and positions.
There is a deep geometrical association among the directions of waves that impede positively, which include the “diffraction pattern,” and crystal structure of the substance. The diffraction configuration is a spectrum of real space periodicities in a material. Diffraction experimentations are important in finding the crystal structures of materials. More evidence concerning a material is enclosed in its diffraction shape. Crystals with accurate periodicities above extended distances have clear and sharp deflection peaks. Crystals having faults including planar faults, impurities, internal strains, dislocations, and small precipitates are not precisely periodic in their atomic arrangements, rather, still possess dissimilar diffraction points. These points are widened, distorted, weakened. Usually, “diffraction line shape analysis” is an imperative mode for study of crystal flaws. Diffraction testing is useful in the study of arrangement of unstructured materials, despite the notion that their diffraction patterns do not give sharp diffraction points.
Scale dissolution test
Acidizing fluid designs – The regular acid systems
The regular acid systems are the acidizing fluid systems whose major constituent is the HCl acid. These were used for the scale dissolution test. Their formulation with other chemicals such as surfactants, iron control agents, etc., make them suitable for stimulation operations in carbonate reservoirs. The recipe is presented in Table 1.
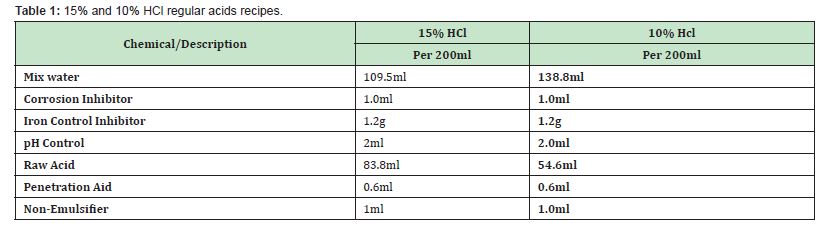
The second stage on scales is the dissolution test which is simply a dissolution test where by treatment fluids designed are tested on sample scales to again identify the scale types and also to test the fluid effectiveness to dissolve the scales. Dissolution rate of selected fluids will be modelled as applicable. The treatment fluid (acid type) used is based on the formation type near the wellbore. In this method, sample scales are weighed into separate beakers and treatment fluids are measured and poured on them for observation.
Scale identification procedure
The scale identification was executed using water, xylene, diesel and acid solutions; 15% HCl and 10% regular HCl solutions.
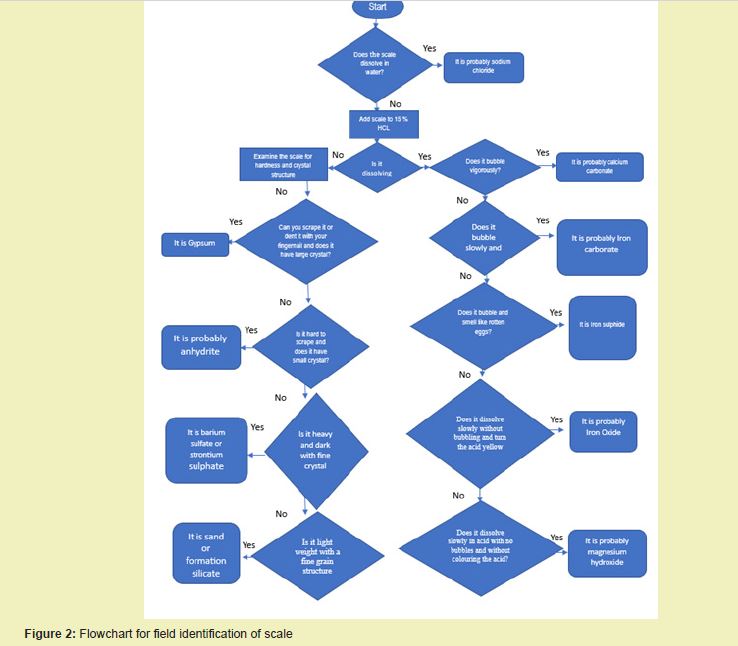
Five beakers each 100 ml were taken and cleaned to get rid of dirt interference with the experiment. About 1 g of the oilfield scales sample was weighed with an electronic balance and placed separately into five beakers and each was well labelled. A 25ml of the prepared 15% HCl and 10% HCl acid mix were separately placed into two of the beakers containing weighed scale samples. Also, 25ml of the xylene, diesel and water were also measured and poured into the three remaining scale samples contained in separate beakers. The experiments were then observed and changes from the beakers containing the different fluids/solutions and scales were recorded. Observations were made as regard the physical parameters (colour change, bubbling, etc.) of scales in their corresponding fluids/solutions. Results are presented under the results and discussion section. The guidelines for field identification of oilfield scales are also offered in the chart in Figure 2 whereas Figure 1 is the XRD Spectral Analysis or a Diffractogram of the mineralogical composition of the scale.
Results description for X-Ray Diffractometry (XRD)
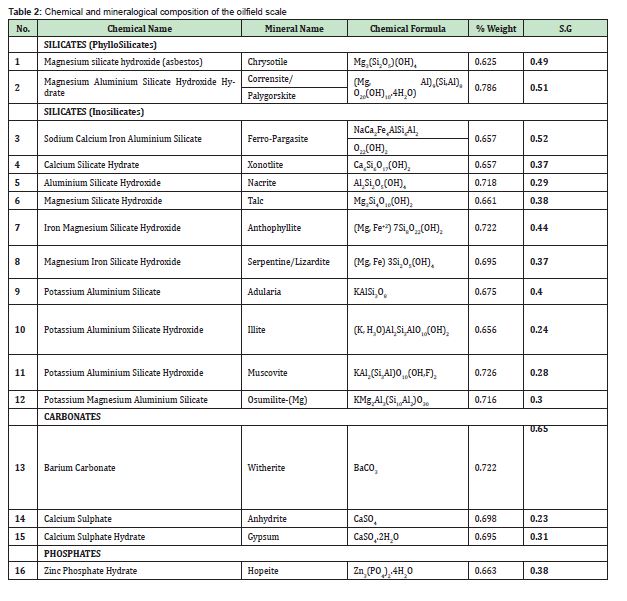
X-ray diffractometry was done for mineralogy identification of scale. The X-ray diffractograms are given in Figure 1. The inferred mineral composition which forms the diffractograms are presented in Table 2.
Results description of scale dissolution analysis
Scale dissolution test was done to identify the scale type and also determine which among the treatment fluid systems has the ability to dissolve the type of scale identified. The fluids used for this scale identification and dissolution analysis are water, diesel, xylene, and 10% and 15% HCl acid mix fluids. Table 3 presents the observations and inferences drawn from the test. Inferences drawn from the observations in Table 3 were guided by Figure 2, which constitutes the field identification strategy for oilfields scales.
X-Ray diffractometry test
From Table 2 the X-ray diffractometry reveals seventeen different kinds of minerals which make up the composition of the sample scale. The minerals identified can be classified as acid soluble and acid insoluble compounds. There are carbonate constituents which is mainly barium carbonate. The silicates constituents are Magnesium silicate hydroxide, lithium aluminium silicate, magnesium aluminium silicate hydroxide hydrate, sodium calcium iron aluminium silicate, calcium silicate hydrate, magnesium silicate hydroxide and iron magnesium silicate hydroxide. The rest of the constituents of the silicates are magnesium iron silicate hydroxide, potassium aluminium silicate, potassium magnesium aluminium silicate, potassium aluminium silicate hydroxide (illite), Potassium aluminium silicate hydroxide (muscovite) and aluminium silicate hydroxide. The sulphates constituents are calcium sulphate hydrate (Gypsum) and calcium sulphate (Anhydrite). The phosphates constituents include the zinc phosphate hydrate.
The acidizing fluids suitable for dissolution of the scale are HCl for the carbonates constituents and HF with its derivatives for the silicate. The sulphates will be inert to acidization.
Scale deposits might be made of many different minerals beside other substances like residues of hydrocarbon and sands. Scale types include those that are acid soluble, acid insoluble and others which comprise of the iron scales and the exotic scales. The acid insoluble scale entails sulphate compounds of Strontium, Barium, and Calcium. Sulphate ions (SO42+) are present in seawater and they undergo reactions with the ions, originally located in formation water subject to the field’s geological history. Acid soluble scales are mostly carbonate compounds of Ca2+ ion; of which CaCO3 is the commonest. Iron scale consists of iron carbonates and iron sulphides whiles exotic scales includes sulphides of zinc and lead. The mixtures result from corrosion of metal parts. Other types of scales comprise silicates; related with injected water and salts scale like NaCl also related through injection of CH3OH.
The mineral elements revealed by the XRD analysis comprise of all these kinds of elements forming various categories of scales listed under Table 2; the carbonates, sulphates of barium compounds, the silicates hydrates and hydroxides compounds magnesium, aluminium, potassium, iron, calcium, zinc, etc.. The silicates compounds are the majority whiles the phosphates, carbonates and sulphates are in minor compositions.
Scale dissolution test
- i. Water
From the results given in Table 3, no noticeable reaction between the sample scale and fresh water. This results from the reason that the sample scale is water insoluble.
- ii. HCl Acid
It was noticed in 15% HCl and 10% HCl that, sample scales had broken into smaller particles. It was then found that lighter particles suspended on the surface whiles the heavy particles were found settling at bottom solution. Thus, HCl acted on the carbonates leaving the other minerals like the silicates and the sulphates undissolved. Observations revealed that larger percentage of scale sample are not soluble in the prepared acid mix (HCl).
Subjecting these samples to temperature of 140 °F (60 °C) using water bath, oil begun separating from sample scales and adhered to walls of the beaker. Inference can be drawn that, the scale at its formative stage in the wellbore, was completely dissolved in the crude oil. In conclusion, sample scale precipitated from the crude oil when there were changes in in-situ reservoir temperature.
- iii. Xylene
Addition of xylene to the scale sample, revealed a colour change of solution, gradually to dark-brownish. This colour change can be credited to crude oil forming on paraffin deposit in the wellbore. It was also clear the quantity of xylene solution had reduced drastically due to volatilisation. Inferences can be made that, if xylene is used as a treatment fluid, it will evaporate with time.
The XRD revealed certain percentage composition of carbonate scales from the mineralogical analysis, but the dissolution rate observed is not appreciable since the carbonates compounds are in minor quantity compared to other compounds (silicates, etc.). This notwithstanding, the major compounds of the minerals present in the scale sample are not acid soluble and thus could not be dissolved.
On scale dissolution, the test did not reveal any mineral in particular but from the observations recorded for xylene and diesel, such as droplets of oil being found on their respective fluids proves the scales are organic and probably contains wax or asphaltenes. The 10% and 15% HCl acidizing fluids could not reveal any visible reaction whiles in case of water, there was no reaction. The XRD analysis carried out on same scale sample reveal several compositions of minerals present such as the Barium carbonate, the hydroxides of mostly the divalent compounds such as Magnesium, the silicate group and iron family scales. The sulphate compounds are acid insoluble thus, a different technique should be used to remove or dissolve them. The carbonate compounds which are acid soluble are present in smaller quantities unlike the sulphate compounds, the silicates and the iron scales. XRD is therefore a suitable method for mineralogical composition determination of wellbore scale. Therefore, XRD results can help design appropriate removal/treatment method without going straight ahead with chemical dissolution test for identification.
None.
This Research Article received no external funding.
Regarding the publication of this article, the authors declare that they have no conflict of interest.

