Aim of this work is to verify the state of the art related m R.N.A. purification process , the technology used as well as the materials employed. New methods vs classic methods: the use of affinity cromato-graphy or and high‐gradient magnetic separation (H.G.M.S.). In particular the use of magnetic beads since introduction of the graphene coated ones. Various producers provide many kind of magnetic beads with various grade of efficiency in separation. Becasue today there is a great debate around graphene derivates peresence (or not) in vials of m R.N.A. VACCINE it is relevant to better clarify the production process as well and the materials used.
This is of toxicological interest.
Keywords: m R.N.A. Vaccine, Production, Purification, Affinity chromatograpy, Magnetic beads, Magnetic beads graphene coated, Biopharmaceutical, Large scale
During last periods in order to fight Covid-19 pandemia was introduced with emergency autorization a new class of vaccine : The m R.N.A. vaccine. This innovative biopharmaceutical products was developed starting form the research in the previous decade related oncologic vaccine research. It is interestig so verifying the manifacturing process used in example to purify this molecule from the impurities or other non useful component. The classic method used solvent , but in order to increase efficiency of the process was introduced purification trough chromatographic methods ( in example affinity cromato-graphy and high efficiency methods With high‐gradient magnetic separation (H.G.M.S. ) and other ). In this process are also used magnetic beads and in order to increase efficiency of interest to verify the role played by the more recent Graphene coated magnetic beads. Because related the production some m R.N.A. VACCINE it is not officially clarify the complete manifacturign process ( probably due to Patents or other industrial secrets)it is crucial to list relevant literature about this argument ( for regulatory reason and toxicological aspect). Various independ reseacher reported in some vials of m R.N.A. VACCINE or other covid-19 vaccine particle like graphene inside and various reseach reported similar particle in blood of vaccinated people. The fact that using graphene magnetic beads make possibile to obtain 170% in efficiency in R.N.A. purification (VS CALSSIC METHODS) is of great interest. This expecially because pharmaceutical producers search great results in production and versus the competitors to have more efficient methods is relevant. In this work are reported a collection of reference involved the keyworks choosen. All article comes from scientific data base.
While the choice of the extraction method can sometimes depend upon the sample type, generally these 3 approaches can be used with most samples
The phenol/chloroform method
This is the classic oldest, tried-and-true method for extracting R.N.A. It uses a straight-forward yet laborious protocol that relies on the differing solubilities of molecular species into organic solvents and water. The phenol/chloroform method can accommodate larger samples, but it is low throughout and not easily automated. Incubation times and precipitation temperatures are important, says M. Beretta, molecular biologist , he uses low temperatures to protect the R.N.A. from degradation by R.N.A.ses. “Attention should be paid not to disturb the phases formed during the process (to prevent contamination of R.N.A. with D.N.A or proteins), so a good handling ability is required,” “The phenol/chloroform method usually yields a slightly cleaner R.N.A. and allows the extraction of R.N.A. from a lower amount of cells [compared to the spin column method- below reported.”
The spin column/column chromatography method
This method uses small centrifuge tubes that contain silica-based filter columns. Lysed samples treated with R.N.A.se inhibitors and high salt concentrations are passed through the spin columns during centrifugation, while the R.N.A. remains bound to the silica within the column. Washing removes impurities, then the purified R.N.A. is eluted from the silica filter using water. Spin columns, widely available in a convenient kit format, typically use a simple, quick protocol. But this can be complicated by incomplete sample lysis / homogenization, or overloaded filters. “It is crucial to use an appropriate amount of input material since using too much sample may reduce lysis efficiency, introduce excessive amounts of cellular components other than R.N.A., and compromise R.N.A. binding to the R.N.A. purification column,” says D. Freedman, senior product marketing manager at NEB. Automation is possible, but it is not as convenient as automating the magnetic -beads method.
The spin column method is only suitable for the small samples, but larger amounts of R.N.A. (in ex grams to kg) can be purified by column chromatography using various resins. For column chromato-graphy based methods, says Kelly Flook, senior product manager at Thermo- Fisher Scientific, “the most important step in the purification workflow is optimizing load and elution steps for the best capacity and yield while maintaining high purity.”
Optimizing salt concentrations is particularly important when purifying mR.N.A. by column chromatography. At higher salt concentrations, mR.N.A. can precipitate or form higher-ordered structures like dsR.N.A.. “When developing loading protocols for oligo(dT) affinity (columns,) it is helpful to first determine the salt concentration at which the mR.N.A. precipitates or forms double strands, [as] loading below this concentration aids recovery and purity,” says Flook. “The level of salt required to bind to oligo(dT) is related to the number of nucleotides that need to be neutralized.”
The magnetic beads method
This method uses magnetic beads coated with silica or other ligands that bind R.N.A., such as oligo(dT) to isolate mR.N.A. molecules with intact polyA tails. The beads are incubated with cell lysate and R.N.A.se inhibitors, then anchored in place using a magnetic field while the supenatant (containing unwanted debris and impurities) is removed, and the beads are washed to remove lingering impurities. Resuspending the beads in water elutes the purified R.N.A. This is a quick, simple protocol and easy to automate for high-throughput work. The lack of a filter column obviates concerns over filter clogging or over-loading. More viscous samples can pose a challenge because it may be more difficult for beads and R.N.A. to make contact during incubation”. In article Journal List Magnetic Separation in Bio-processing Beyond the Analytical Scale: From Biotechnology to the Food.
“Down-stream processing needs more innovative ideas to advance and overcome current bio-processing challenges. Chromato-graphy is by far the most prevalent technique used by a conservative industrial sector. Chromato-graphy has many advantages but also often represents the most expensive step in a pharmaceutical production process. So, alteR.N.A.tive methods as well as further processing strategies are urgently needed. One promising candidate for new developments on a large scale is the magnetic separation, which enables the fast and direct capture of target molecules in fermentation broths. There has been a small revolution in this area in the last 10–20 years and a few papers dealing with the use of magnetic separation in bio-processing examples beyond the analytical scale have been published. Since each target material is purified with a different magnetic separation approach, the comparison of processes is not trivial but would help to understand and improve the magnetic separation and thus making it attractive for technical scale. We report, here, on the latest achievements in magnetic separation technology and offer an overview of the progress of the capture and separation of bio molecules derived from biotechnology and food technology. The Magnetic separation has great potential for high-throughput down-stream processing in applied life sciences. At the same time, 2 major challenges need to be overcome:
- 1. The development of a platform for suitable and flexible separation devices
- 2. Additional investigations of advantageous processing conditions, especially during recovery
Concentration/purification factors need to be improved to pave the way for the broader use of magnetic applications. The innovative combination of magnetic gradients and multipurpose separations will set new magnetic-based trends for large scale downstream processing. Magnetic- separation is an interesting candidate for future down-stream applications due to some important advantageous features:
- 1. Integrated 1 step capture and purification of target (high affinity and selectivity)
- 2. High throughput
- 3. Semi-continuous processing with low energy consumption
Thus, magnetic separation can help reduce costs and increase yields and productivity compared to traditional processes.
The webinar will review the basic concepts of magnetic -separation and help the attendees understand how advanced systems may enlight key aspects of the process. These concepts will be applied to parameterize, monitor and validate the magnetic beads behavior in controlled conditions. The discussion will focus on how to transfer the correctly characterized bio-magnetic separation process from laboratory to production scale Figure 1&2.
Viral Nucleic Acid Extraction Magnetic Microspheres
Description
VDO biotech’s magnetic microsphere for viral nucleic acid is a core-shell structure and polydisperse magnetic microsphere, and the surface is coated with silicon hydroxyl groups. MS02HA has fast magnetic response speed, excellent suspension, and outstanding hydrophilicity and nucleic acid capture ability. MS02HA is highly efficient in extracting nucleic acids from virus, pseudovirus particles and small fragments. It is suitable for a variety of sample types, suitable for automated nucleic acid extraction, and is an ideal choice for biological sample purification. Advantages:
- a. Large specific surface area: The high capability to combine with nucleic acid
- b. High hydrophilic property: Reduce the possibility of the beads adhering to the tube walls
- c. Multiple sample compatibility: Are suitable for the virus, pseudoviral and small nucleic acid fragment
- d. Special chemical groups: The high capacity to adsorb nucleic acid and ease to elute
Affinity ligand modified magnetic microspheres
Affinity ligand magnetic microspheres have uniform particle size, stable and controllable surface functional groups, and excellent experimental repeatability. They can be used for high-throughput purification, and can directly obtain high-purity target protein from coarse samples. In addition, VDO Biotech provide customized microspheres with different particle sizes and surface functional groups to meet the special purification requirements of different samples and applications.
Oligo(dT) Magnetic Microspheres
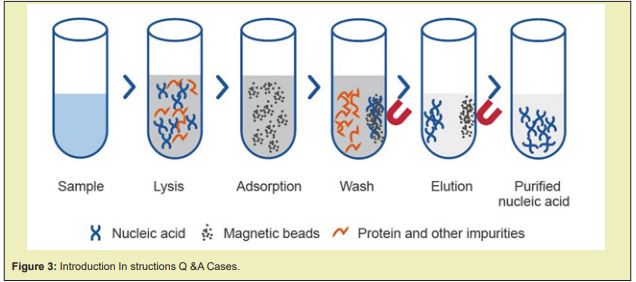
Introduction instructions Q&A cases
Oligo(dT) Magnetic Microspheres are applicable to rapid isolation of high purity complete mRNA from total RNA of eukaryotic cells or from crude extracts of cell, animal, and plant tissues. Isolated mRNAs can be directly used in most downstream applications of molecular biology, such as RT-PCR, solid-phase cDNA library construction, S1 nuclease analysis, ribonucleic acid protection analysis, primer extension, spot hybridization, in vitro translation experiments, RACE, cut-back hybridization, Northern analysis, gene cloning, and gene expression analysis Figure 3-5.
Comparison of Three Magnetic Bead Surface Functionalities for RNA Extraction and Detection
Magnetic beads are convenient for extracting nucleic acid NA biomarkers from biological samples prior to molecular detection. These kind of beads are available with a variety of surface functionalities designed to capture particular subsets of RNA. We hypothesized that bead surface functionality affects binding kinetics, processing simplicity, and compatibility with molecular detection strategies. In this report,3 magnetic bead surface chemistries designed to bind nucleic acids, silica, oligo (dT), and a specific oligo-nucleotide sequence were evaluated. Commercially available silica-coated and oligo (dT) beads, as well as beads functionalized with oligo-nucleotides complementary to respiratory syncytial virus (R.S.V) nucleo-capsid gene, respectively recovered ∼75, ∼71, and ∼7% target RSV m-RNA after a 1 min of incubation time in a surrogate patient sample spiked with the target. RSV-specific beads required much longer incubation times to recover amounts of the target comparable to the other beads (∼77% at 180 min). As expected, silica-coated beads extracted total R.N.A, oligo (dT) beads selectively extracted total mRNA, and RSV-specific beads selectively extracted RSV N gene mRNA. The choice of bead functionality is generally dependent on the target detection strategy. The silica-coated beads are most suitable for applications that require nucleic acids other than m-RNA, especially with detection strategies that are tolerant of a high concentration of nontarget background nucleic acids, such as RT-PCR. On the other hand, oligo (dT) beads are best-suited for mRNA targets, as they bind bio markers rapidly, have relatively high recovery, and enable detection strategies to be performed directly on the bead surface. Sequence-specific beads may be best for applications that are not tolerant of a high concentration of nontarget nucleic acids that require short RNA sequences without poly(A) tails, such as microRNAs, or that perform RNA detection directly on the bead surface .Recently, silica-coated magnetic iron oxide MNPs (MNPs@SiO2) have been successfully developed and used in biomedical applications, such as macromolecular separation. For application, GO has been shown to be a suitable platform for protein and nucleic acid adsorption.
VT mRNA Purification -Services
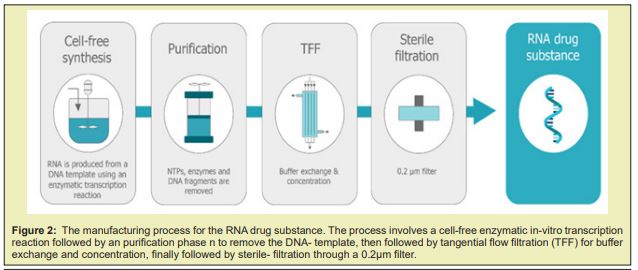
There has been great interest around in vitro-transcribed (IVT) mRNA for the research and the therapeutic applications in recent years. The manufacturing of pure mR.N.A. products are on-demand as the importance of IVT m-RNA is gradually rising. With various years of experience in IVT mR.N.A. synthesis and purification technology, Creative Biogene has developed robust, and cost-effective protocols to purify IVT mRNA to help world-wide customers obtain efficient and high-quality synthetic mRNAs Figure 6.
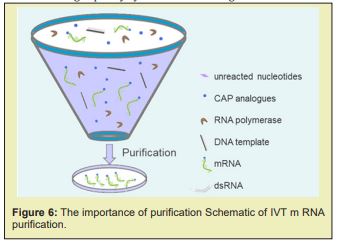
The purity of IVT mR.N.A. is a key factor in determining the efficiency of target antigen AG expression. After in vitro transcription, the reaction solution contains m RNAs and remaining reagents. The remaining reagents of the in vitro synthesis process are comprised of R.N.A. polymerase, un-reacted nucleotides, CAP analogues, DNA template as well as double-stranded RNA (dsRNA) side products. Previous studies have identified that the presence of certain by-products can trigger cellular immune responses. Thus, purifying mRNA from these contaminant impurities is critical, especially for in vivo- applications. Creative Biogene' IVT mRNA purification protocols Various purification techniques have been developed to enhance the concentration and quality of IVT mRNA, including high-performance liquid chromatography (H.P.L.C.), fast protein liquid chromatography (FPLC) and magnetic- beads technology.
HPLC purification of IVT mR.N.A
For large-scale purification and isolation, HPLC is most often used. Previous studies demonstrated that the translation efficiency of HPLC-purified mRNA is greatly enhanced. H.P.L.C. can be used to isolate synthesized mRNA from the remaining reagents via size exclusion or ion-exchange columns, thereby removing smaller or larger by-products, such as dsRNA, abortive transcripts and so on.
mRNA purification using the magnetic beads technology
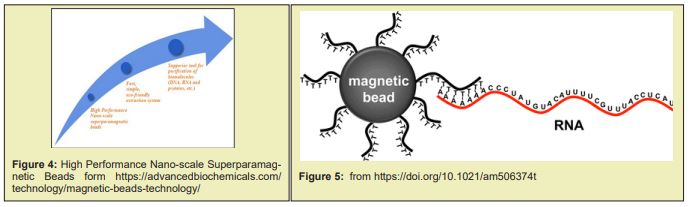
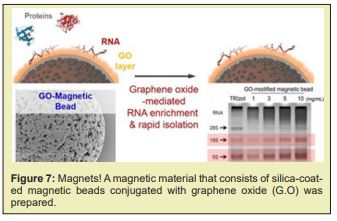
the Magnetic-beads are made up of tiny (20 to 30nm) particles of iron oxides, which give them superparamagnetic properties. In contrast to prior purification methods, this method is fast, consistently reliable, and the most efficient in m RNA purification. Under optimized conditions, m R.N.A. selectively binds to the surface of magnetic- beads, while other contaminants stay in in vitro transcription reaction solution”. A magnetic material that consists of silica-coated magnetic beads conjugated with graphene oxide (G.O) was successfully prepared for a facile ribonucleic acid (RNA) extraction. When the G.O-modified magnetic beads were applied to separate the RNA from the lysed cell, the cellular RNAs were readily adsorbed to and readily desorbed from the surface of the G.O-modified magnetic beads by urea. The amount of R.N.A. extracted by the G.O-modified magnetic beads was ≈170 % as much as those of the control extracted by a conventional phenol-based chaotropic solution. These results demonstrate that the facile method of RNA separation by using G.O-modified magnetic beads as an adsorbent is an efficient and simple way to purify intact cellular RNAs and/or microRNA from the cell –lysates Figure 7.
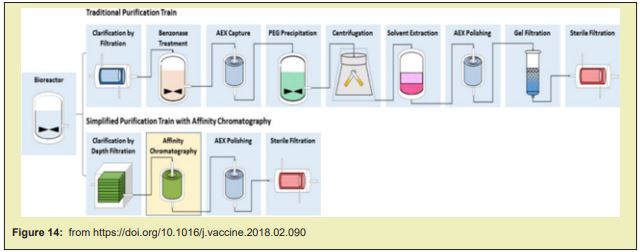
A magnetic material that consists of silica-coated magnetic beads conjugated with graphene oxide (G.O) was prepared. The material was tested in the separation of ribonucleic acid (RNA) in a lysed- cell, and the results show that the G.O-modified magnetic beads can be used as mobile solid-phase particle candidates, and used to directly separate the R.N.A. in bio-materials. The increased demand for mR.N.A. vaccines requires a technology platform and cost-effective manufacturing process with a well-defined product characterisation. Large- scale production of mR.N.A. vaccines consists in a 1 or 2-step in vitro reaction followed by a purification platform with multiple steps that can include Dnase digestion, precipitation, chromatography or tangential- flow filtration. mR.N.A. is produced in a cell-free system and uses no animal derived raw materials. Cell-derived impurities or adventitious contaminations are thus absent, which makes the manufacturing of these molecules safer. Although several commercial kits are available to produce mR.N.A. for preclinical studies at laboratory scale, their costs are high. The generation of mR.N.A. by IVT at large- scale and under current good manufacturing -practice (cGMP) conditions is also challenging. The specialised components of the IVT reaction must be acquired from certified suppliers that guarantee that all the material is animal component-free and G.M.P.-grade. The availability of large amounts of these materials is limited and purchasing costs are high. This is true, for example, in the case of the enzymes used for translation and capping. Once the mR.N.A. is generated by IVT, it must be isolated and purified from the reaction- mixture using multiple purification steps to achieve clinical purity standards. The reaction mixture contains not only the desired product, but also a number of impurities, which includes enzymes, residual NTPs and D.N.A template, and aberrant mR.N.A.s formed during the IVT. Traditional lab scale purification methods are based on D.N.A. removal by DNAse digestion followed by lithium chloride (LiCl) precipitation.These methods do not allow the removal of aberrant m-RNA species such as dsRNA and truncated R.N.A fragments. The removal of these product-related impurities is crucial for mR.N.A. performance, as they lower translation efficiency and modify the immuno-stimulatory profile. For example, a 10–1000-fold increase in protein production was observed when nucleoside-modified mR.N.A. was purified by reverse phase H.P.L.C. prior to delivery to primary DC. Chromato-graphy is a mainstream purification process widely accepted in the pharmaceutical industry. Its high popularity is derived from several attributes such as selectively, versatility, scalability and cost-effectiveness. The first published protocol for large scale purification of synthetically produced RNA oligo-nucleotides used size exclusion chromatography (S.E.C) in a gravity-flow mode to separate molecules according to size. Further studies work applying SEC with fast performance liquid -chromatography were performed. These techniques allowed a preparative scale purification -process, achieving high purity and high yields. SEC presents limitations, as it is not able to remove similar size impurities, such as dsD.N.A.
The use of ion pair reverse-phase chromatography (I.P.C) proved to be an excellent method for mR.N.A. purification. In IPC, the negatively (-) charged sugar-phosphate backbone of the oligo nucleotides will pair with quaternary- ammonium compounds QUATS present in the mobile phase to become lipophilic and then interact with the stationary phase of a reverse-phase chromato-graphy column. Elution is then performed with a gradient of an adequate solvent, (like acetonitrile). Using this approach, dsRNA impurities are effectively removed while maintaining the process's high yield. IPC is challenging and costly to scale, and the use of toxic reagents such as aceto-nitrile, is not desirable. A new cellulose-based chromato-graphy process for the removal of dsRNA has been described that leverages the ability of dsRNA to bind to cellulose in presence of ethanol . This method reported a mR.N.A. yield of greater than 65% with a dsRNA removal of over 90%. Still, the removal of other impurities was not addressed, and thus the introduction of pre-purification steps is likely to be required. Ion exchange chromatography (IEC) can also be used to purify mR.N.A. at large scale. This technique explores the charge difference between the target mR.N.A. species and the different impurities.In ex, weak anion exchange chromato-graphy has been successfully implemented to separate mR.N.A. from IVT impurities. IEC presents several advantages: it is scalable and cost-effective; it allows the separation of longer R.N.A transcripts; and it presents higher binding capacities (when compared to IPC).This chromatography must be performed under denaturing conditions. This makes the process more complex as it requires a mobile phase heater and a tight -control of the temperature during chromato-graphy.
Affinity based separation is another mR.N.A. purification approach. A single-stranded sequence of deoxythymidine (dT) - Oligo dT - is routinely used for the capture of mR.N.A. in lab. applications. This sequence binds to the poly-A tails present in the mR.N.A.. Chromatographic- beads with immobilized oligo dT could thus be used for the process scale purification using affinity chromato graphy: the poly-A tails of the single stranded mR.N.A. produced during IVT would bind to the stationary phase while impurities are washed out. This way, IVT un-consumed reagents, the D.N.A template and dsRNA could be efficiently removed. While high purity products can be obtained using affinity chromatography, several draw-backs are present such as low binding capacities and a less cost-effective process. The removal of the small size impurities can also be achieved while concentrating or diafiltrating solutions by tangential flow filtration (TFF). Core bead chromato-graphy can also be used for this purpose. In this case, small impurities are trapped inside the beads, and the product will be in the flow-through.Both techniques rely on DNase- digestion or denaturing agents to remove high size molecules such as the DNA template or the polymerase. D.N.A removal can also be achieved using hydroxyapatite chromato-graphy without the use of a DNase. As a polishing step, hydrophobic interaction chromatography (HIC) can be applied using connective interaction media monolith containing OH or SO3 ligands.
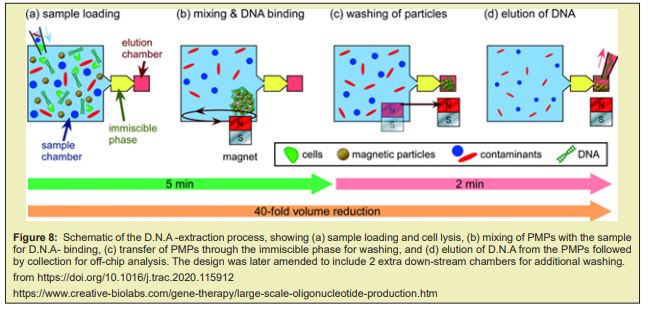
Large scale adaptations of the traditional lab scale mR.N.A. purification methods are also being explored. The mR.N.A. precipitation can be combined with TFF technique. During TFF, the membrane captures the precipitated mR.N.A. product while other impurities are removed by dia-filtration. The product is then eluted by re-solubilizing the mR.N.A.. D.N.A template removal can be achieved by performing the digestion with immobilised DNase. Other approach is to use tagged DNA- template that can then be removed after IVT using affinity- chromatography AC. Despite being scalable, these methods present a limited effectiveness since they only focus on removal of some specific impurities and hence must be coupled with other purification steps Figuer 8.
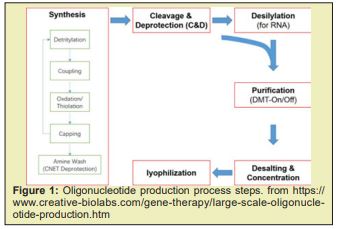
Large Scale D.N.A. and R.N.A. Oligonucleotide Production Services
Synthesis: Solid-phase synthesis has been employed for the manufacture of oligonucleotides from micrograms for research use. During the solid-phase synthesis, phosphoramidite monomers are added sequentially onto a solid support to generate the desired full-length oligo-nucleotide. Each cycle of base addition consists of 4 chemical reactions, detritylation, coupling, oxidation/thiolation, and capping. Cleavage and Deprotection (C&D): Oligo-nucleotide de-protection involves 3 steps: removal of a cyanoethyl protecting group from the phosphate backbone, cleavage of the oligonucleotide chain from the support, and base deprotection. This process is generally carried out in batch mode where the entirety of the solid support is incubated with C&D reagents. Purification: The purification of oligonucleotide crude solutions is generally achieved by chromatographic methods. Taking advantage of the internucleotide phosphodiester and phosphorothioate linkage, anion exchanged and ion-paired reversed-phase (IP-RP) chromatographic purifications on HPLC equipment are widely used for the purification of the therapeutic oligonucleotide.
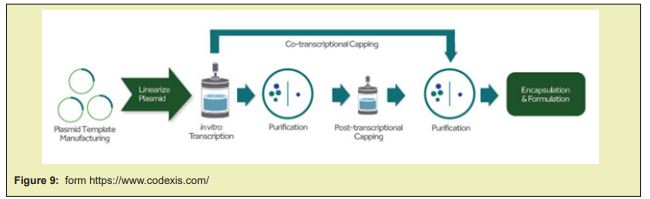
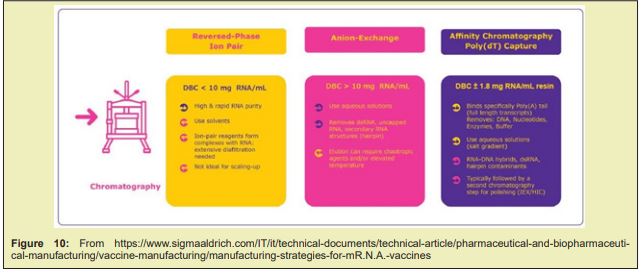
Desalting and Concentration: The most widely accepted choice for therapeutic oligonucleotide isolation at manufacturing scales is the use of tangential flow filtration (TFF). This approach employs an appropriately sized filter membrane and uses a pump to circulate the sample through the TFF set-up making the process more efficient. Lyophilization: This step is performed using a freezer dryer, which is a machine consisting of a sample chamber, a condenser and a vacuum pump. The oligonucleotide is frozen and lyophilized to obtain the final product in powder form via sublimation Figure 9&10.
Simple, flexible, and scalable mR.N.A. production
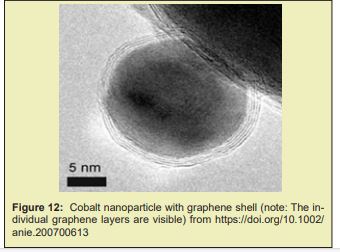
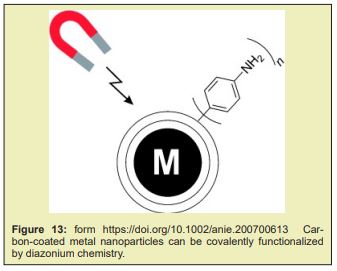
Accelerating the development and manufacturing of mR.N.A. vaccine and therapeutics requires simple workflows with all inputs optimized for product needs. The demand for high-quality mR.N.A. purification and synthesis must be met with flexible and scalable production solutions. Invitrogen Dynabeads Streptavidin for In- Vitro Transcription and Invitrogen Dynabeads Carboxylic Acid for RNA Purification are specifically designed for optimal mR.N.A. production. Turbobeads made from highly reactive metal nanomagnetics that are coated in graphene carbon. The combination of the metal core with the carbon shell displays a large increase in magnetic properties; allowing for faster separation Figure 11-13.” Inorganic coating, such is the case of silica (which allows functionalization) [26,104,110,124], graphene (granting better thermal stability and electrical conductivity and larger surface-to-volume ratio to further the potential array of applications) [110] and gold (allowing functionalization, biocompatibility and protecting MNPs from oxidation) [26,104,110] have also been used.

Nhs Mag Sepharose, Sera-Mag Carboxylate-Modified, Sera-Mag Speedbeads Carboxylate-Modifi
Preactivated magnetic beads are designed for covalent coupling of antibodies, aptamers, and proteins. After coupling is performed, proteins of interest can be affinity captured and enriched using immunoprecipitation . The preactivated magnetic- beads include NHS Mag Sepharose, Sera-Mag Carboxylate-Modified, and Sera-Mag SpeedBeads Carboxylate-Modified Magnetic Particles https://www.cytivalifesciences.com/en/us/solutions/genomics/knowledge-center/magnetic-bead-guide/mag-beads-for-protein-purification. Magnetic beads are used to purify single proteins, large protein complexes, antibodies and for high-throughput purifications. Magnetic beads based on chromato-graphy resins provide functions of the resin with the convenience and ease-of-use of magnetic beads”.
Ribonucleic acid purification
Disclosed herein are methods for purifying R.N.A. comprising poly A. Also disclosed herein are compositions such as surfaces and oligonucleotides for purifying R.N.A. comprising polyA. Other embodiments are also disclosed. Commercially-available resins having polythymidine oligonucleotide ligands typically contain less than 30 thymidine (2'deoxy) residues and some commercial resin suppliers utilize a distribution of dT chain lengths, not of a discreet length. Commercially-available matrices typically consist of cellulose, latex particles, and magnetic beads containing dT ligands”.
Purification of mR.N.A. with oligo(dT)-functionalized magnetic particles involves a series of magnetic separations for buffer exchange and washing. Magnetic particles interact and agglomerate with each other when a magnetic field is applied, which can result in a decreased total surface area and thus a decreased yield of mR.N.A.. In addition, agglomeration may also be caused by mR.N.A. loading on the magnetic particles. High-gradient magnetic separation (H.G.M.S.) can be used to selectively separate magnetizable components from suspensions. This technique has already been applied by various working groups in the field of biotechnology as well. It was utilized for the separation of immobilized enzymes and used for the isolation of target molecules. In this process, the target molecule is specifically adsorbed on the functionalized particle surface in a reaction mixture and desorbed again from the magnetic particles (named magnetic beads) after magnetic separation has taken place. An overview of widespread magnetic separators and magnetic particle systems is given in. One widely used micro particle system in the bio separation of proteins, mR.N.A., and viruses is Dynabeads pharmacy is the production of mR.N.A. for vaccine manufacturing. The synthesis of a mR.N.A. vaccine is described in the literature. Within the production of a vaccine, separation and purification of mR.N.A. are necessary in the process.
Practical Application
Magnetic microparticles have gained importance
in the purification of mR.N.A.-based vaccines. They serve as adsorbents for the mR.N.A.. Through several steps of magnetic separation followed by redispersion of the magnetic beads for washing and elution, the mR.N.A. can be isolated. This is usually done on a mL scale. To obtain larger amounts of mR.N.A., flow-through magnetic separation using high-gradient magnetic separation (H.G.M.S.) can be advantageous. Here, the suspension to be processed is then usually in a stirred feed tank. Due to particle-particle interactions, a not inconsiderable agglomeration may occur, especially due to the attached mR.N.A.. This can reduce the mR.N.A. yield. For economic reasons it is necessary to perform magnetic particle recycling, for which there are various process alteR.N.A.tives. With regard to a possible use of H.G.M.S. in an mR.N.A. production for vaccines, particle size distributions were determined to investigate the agglomerating or deagglomerating effect of different process steps”
Related the topics of this work variuos relevant reference are reported , all article comes from PUBMED or other scientific database. After this review part and ecperimental project hypothesys is submitted to the reseacher in order to provide a global conclusion related to the aim of the work.
From literature
Therapeutic cancer vaccines require a -robust cellular immunity for the efficient killing of tumor cells, and recent advances in neo-antigen discovery may provide safe and promising targets for cancer vaccines. Elicitation of T cells with strong antitumor efficacy requires intricate multistep processes that have been difficult to attain with traditional vaccination approaches. A multifunctional nano vaccine platform has been developed for direct delivery of neo-antigens and adjuvants to lymph nodes (LNs) and highly efficient induction of neoantigen-specific T cell responses. A PEGylated reduced graphene oxide nanosheet (R.G.O.-PEG, 20-30nm in diameter) is a highly modular and bio-degradable platform for facile preparation of neoantigen vaccines within 2h. R.G.O.-PEG exhibits rapid, efficient (15-20% ID/g), and sustained (up to 72h) accumulation in LNs, achieving >100-fold improvement in LN-targeted delivery, compared with soluble vaccines. R.G.O.-PEG induces intracellular reactive- oxygen species in dendritic cells, guiding antigen processing and presentation to T cells. Importantly, a single injection of R.G.O.-PEG vaccine elicits potent neoantigen-specific T cell responses lasting up to 30 days and eradicates established MC-38 colon carcinoma. Further combination with anti-PD-1 therapy achieved great therapeutic improvements against B16F10 melanoma. R.G.O.-PEG may serve a powerful delivery platform for personalized cancer vaccination.1
Messenger R.N.A. (m-R.N.A.) vaccine is a promising candidate in cancer immunotherapy as it can encode tumor-associated antigens with an excellent safety profile. Unfortunately, the inherent instability of R.N.A. and translational efficiency are major limitations of R.N.A. vaccine. We report an injectable hydro-gel formed with graphene oxide (GO) and polyethylenimine (PEI), which can generate mR.N.A. (ovalbumin, a model antigen) and adjuvants (R848)-laden nanovaccines for at least 30 days after subcutaneous injection. The released nanovaccines can protect the m-R.N.A. from degradation and confer targeted delivering capacity to lymph nodes. The data show that this transformable hydro-gel can significantly increase the number of antigen-specific CD8+ T cells and subsequently inhibit the tumor growth with only 1 treatment. This hydro-gel can generate an antigen specific antibody in the serum which in turn prevents the occurrence of metastasis. Collectively, these results demonstrate the potential of the PEI-functionalized GO transformable hydro-gel for effective cancer immuno-therapy.2
The Gene -therapy is emerging as a valid method for the treatment of ovarian cancer, including small interfering R.N.A. (siR.N.A.). Although it is so powerful, few targeting efficient gene delivery systems seriously hindered the development of gene therapy. In this research work study, we synthesized a novel gene vector PEG-GO-PEI-FA by functionalized graphene oxide (G.O), in which folic acid can specifically bind to the folate receptor (FR), which is overexpressed in ovarian cancer. Characterizations of the nanocomplexes were evaluated by dynamic light scattering (DLS), atomic force microscopy , and Fourier transform infrared spectroscopy (F.T.I.R.). The siR.N.A. condensation ability and stability were assessed by agarose gel electrophoresis. Cellular uptake efficiency and lysosomal escape ability in ovarian cancer cells were investigated by confocal laser scanning microscopy. Cellular bio safety of the system and inhibitory of the siR.N.A. tolerability were evaluated by CCK-8 assay. The size of the PEG-GO-PEI-FA nanocomplexes was 216.1±2.457 nm, exhibiting mild cytotoxicity in ovarian cancer cells. With high uptake efficiency, PEG-GO-PEI-FA can escape from the lysosome rapidly and release the gene. PEG-GO-PEI-FA/siR.N.A. can effectively inhibit the growth of ovarian cancer cells. By and large, the PEG-GO-PEI-FA/siR.N.A. may offer a promising strategy for siR.N.A. delivery in the treatment of FR-positive ovarian carcinoma or similar tumors.3
Isolated, atomically thin conducting membranes of graphite, called graphene, have recently been the subject of intense research with the hope that practical applications in fields ranging from electronics to energy science will emerge. The atomic thinness, stability and electrical sensitivity of graphene motivated us to investigate the potential use of graphene membranes and graphene nano-pores to characterize single molecules of D.N.A. in ionic solution” Electrical measurements on graphene membranes in which a single nano-pore has been drilled show that the membrane's effective insulating thickness is less than 1 nano-meter. This small effective thickness makes graphene an ideal substrate for very high-resolution, high throughput nanopore-based single molecule detectors.4
Metrics
Graphene Substrates for Drug Delivery, GO with its oxygen-containing functional groups (COOH and OH) has been reported as an effective carrier for drug or gene delivery.5 Clinical applications of induced pluri-potent stem cells require development of technologies for the production of "footprint-free" (gene integration-free) iPSCs, which avoid the potential risk of insertional mutagenesis in humans. Previously, several studies have shown that mR.N.A. transfer can generate "footprint-free" iPSCs, but these studies did not use a delivery vehicle and thus repetitive daily transfection was required because of mR.N.A. degradation. We report an mR.N.A. delivery system employing graphene oxide (GO)-polyethylenimine complexes for the efficient generation of "footprint-free" iPSCs. GO-PEI complexes were found to be very effective for loading mR.N.A. of reprogramming transcription factors and protection from mR.N.A. degradation by R.N.A.se. Dynamic suspension cultures of G.O-PEI/R.N.A. complexes-treated cells dramatically increased the re-programming efficiency and successfully generated rat and human iPSCs from adult adipose tissue-derived fibroblasts without repetitive daily transfection. The iPSCs showed all the hallmarks of pluripotent stem cells including expression of pluripotency genes, epigenetic reprogramming, and differentiation into the 3 germ layers. These results demonstrate that mR.N.A. delivery using GO-PEI-R.N.A. complexes can efficiently generate "footprint-free" iPSCs, which may advance the translation of iPSC technology into the clinical settings.6
“The observations under a pHase Contrast, Dark-Field, BrightField microscopy, Transmission and Scanning Electron microscopy of the vaccine product by Pfizer, including vaccine products of ModeR.N.A., Astrazeneca and Janssen revealed some entities that can be graphene strips as seen below in Figure reported. [Figure reported shows an aqueous fraction image from Pfizer vaccine sample (left) and from reduced graphene oxide (R.G.O.) standard (right) (Sigma-777684). Optical microscopy, 100X] [Figure reported Aqueous fraction images containing reduced graphene oxide from Pfizer vaccine sample (left) and sonicated reduced graphene oxide (R.G.O.) standard (right) (Sigma-777684). Optical pHase contrast microscopy, 600X. The Muestra RD1, La Quinta Columna Report, June 28, 2021; Graphene Oxide GO Detection in Aqueous Suspension; Delgado Martin, Campra Madrid confirmed our findings.] For a definitive identification of graphene by TEM, it is necessary to complement the observation with the structural characterization by obtaining a characteristic electron diffraction standard sample (as the figure 'b' shown below). The standard sample corresponding to graphite or graphene has a hexagonal symmetry, and generally has several concentric hexagons. [Figure reported Reveals X ray Diffraction Pattern of the Graphene Particles.] Using Transmission Electron Microscopy (TEM) we observed an intricate matrix or mesh of folded translucent flexible R.G.O. sheets with a mixture of darker multilayer agglomerations and lighter colored of unfolded monolayers as seen in Figure reported [Figure reported shows the liposome Capsid containing R.G.O. that Pfizer uses for its product to vehiculate the graphene oxide by attaching the Liposome capsid to specific mR.N.A. molecules for driving the Liposome contents off GO to specific organs, glands and tissues, namely the ovaries and testes, bone marrow, heart and brain.
The image was obtained by a SEM-Cryo preparation.] Using (TEM) revealed an intricate matrix or mesh of folded translucent flexible R.G.O. sheets with a mixture of darker multilayer agglomerations and lighter colored of unfolded monolayers as seen in Figure reported [Fig. reported shows a cluster of graphene nano-particles in a Pfizer vaccine. They appear to be aggregated.] The darker linear areas in Figure reported appear to be local overlap of sheets and local arrangement of individual sheets in parallel to the electron beam. After the mesh, a high density of unidentified rounded and elliptical clear shapes appears, possibly corresponding to holes generated by mechanical forcing of the R.G.O. mesh during treatment as seen in Figure reported. [Figure 6 shows a TEM microscopy observation where particles of reduced graphene oxide in a Pfizer” vaccine” are present. The X-ray diffractometry reveals their nature of crystalline Carbon-based nano-particles of R.G.O.. Energy-Dispersive X-ray Spectro-scopy Reveals R.G.O. in Pfizer Vaccine. The Pfizer vaccine liquid fraction was then analyzed for chemical and elemental content using Energy-dispersive X-ray spectroscopy (E.D.S.) as seen in Fig. 6. The E.D.S. spectrum showed the presence of Carbon, Oxygen verifying the R.G.O. elements and Sodium and Chloride since the sample shown in Figures reported were diluted in a saline solution. [Figure rported shows an E.D.S. spectrum of a Pfizer “vaccine” under an ESEM microscopy coupled with an E.D.S. x-ray microprobe (X axis =KeV, Y axis = Counts) identifying Carbon, Oxygen, Sodium and Chloride.] The Quantification of mR.N.A. in the Pfizer Vaccine The quantification of R.N.A. in the Pfizer sample was carried out with conventional protocols (Fisher).
According to NanoDropTM 2000 spectro photometer calibration check specific software (Thermo-fisher), the UV absorption spectrum of total aqueous fraction was correlated to 747 ng/ul of unknown absorbing substances. After R.N.A. extraction with commercial kit (Thermo-fisher), quantification with R.N.A. specific Qbit fluorescence probe (Thermo-fisher) showed that only 6t ug/ul could be related to the presence of R.N.A.7
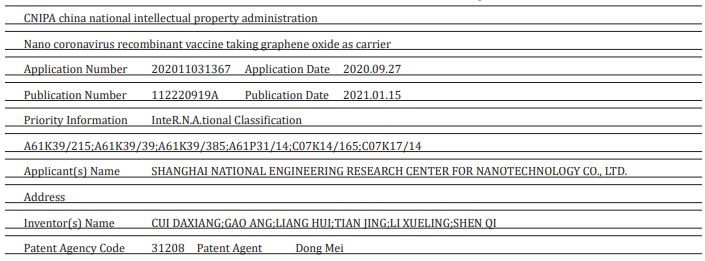
“The invention belongs to the field of nano materials and biological medicines, and relates to a vaccine, in particular to development of a 2019-nCoV coronavirus nuclear recombinant nano vaccine. The invention also comprises a preparation method of the vaccine and application of the vaccine in animal experiments. The novel coronavirus vaccine contains graphene oxide, carnosine, CpG and novel coronavirus RBD; The carnosine, the CpG and novel coronavirus R.B.D are combined on a framework of the graphene oxide; the coding sequence of the CpG is as shown in SEQ ID NO 1; and the novel coronavirus RBDrefers to that a novel coronavirus protein receptor binding region can generate a high-titer specific antibody aiming at the RBD in a mouse body, and strong support is provided for prevention and treatment of the novel coronavirus.8 A magnetic material that consists of silica-coated magnetic beads conjugated with graphene oxide (GO) was successfully prepared for facile ribonucleic acid (R.N.A.) extraction. When the G.O-modified magnetic beads were applied to separate the R.N.A. from the lysed cell, the cellular R.N.A.s were readily adsorbed to and readily desorbed from the surface of the G-O-modified magnetic beads by urea. The amount of R.N.A. extracted by the G.O-modified magnetic beads was about 170% as much as those of the control extracted by a conventional phenol-based chaotropic solution. These results demonstrate that the facile method of R.N.A. separation by using G.O-modified magnetic beads as an adsorbent is an efficient and simple way to purify intact cellular R.N.A.s and/or microR.N.A. from cell lysates. © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.9
The invention relates to a process for separating a dispersed phase from a continuous phase comprising the steps of i) contacting said phases with an effective amount of nano-particles; ii) applying a magnetic field gradient to the obtained system; iii) separating the obtained phases wherein said nano-particles are of the core shell type, said core consists of a metal or alloy having soft magnetic properties and said shell contains a graphene layers which are optionally functionalized; to new nano-particles and method of manufacturing such nano-particles. In yet another application in ex. for food additive production or in biotechnical production of proteins, viruses, vaccines and antibodies the magnetic functionalization of the cells may be used to keep them afloat in a desired volume of liquid allowing the separation of the liquid, containing a desired product from their site of production ( cells, micro-organisms). This application may also be applied in wast-treatment plants.”
Development of a 3D‐printed single‐use separation chamber for use in mR.N.A.‐based vaccine production with magnetic microparticles Lars Wommer,Patrick Meiers, Isabelle Kockler, Roland Ulber, Percy Kampeis. A new variant of vaccine production is becoming increasingly important with regard to viral infectious diseases. This involves the use of mR.N.A. produced by cell culture methods. This mR.N.A. is protected by special formulations and introduced into human cells, where it induces the expression of proteins and thus, triggers the immune response. In the search for new vaccines, lab. protocols that use functionalized magnetic beads for the purification of mR.N.A. out of cell-lysates are often used. For this purpose, magnetic particles with deoxythymidine functionalization (oligo(dT)), with a sequence of 14-25 thymine bases are utilized. Hybridization of the mR.N.A. to the oligo(dT) magnetic particles takes place specifically, due to the base adenine complementary to thymine. Only mR.N.A. molecules have an adenine chain with 40-250 units at the 3′ end. This does not exist on R.N.A. or D.N.A. molecules, so selective sorption occurs by hybridization of mR.N.A. on the oligo(dT) magnetic particles. After separation of the particles loaded with mR.N.A. in the magnetic field and removal of impurities with various washing steps, elution of the mR.N.A. can be initiated by increasing the temperature. Here, successive multiple magnetic separations and resuspensions are involved, for which suitable millilitre‐scale lab. protocols have been developed, some of which are automated.
With the developed SU‐H.G.M.S. separation chamber, mR.N.A. manufacture can be realized at such a production scale, which should be sufficient for clinical trials. The standard operating procedures can be transferred directly from the corresponding lab. protocols using magnetic particles. Increasing the amount of magnetic particles to be processed can be easily achieved by parallelization (up‐numbering). High‐gradient magnetic separation If it was possible to use oligo(dT) magnetic particle‐based purification of mR.N.A. for clinical trials, there would be no need for a technology change in scale‐up from lab. scale to process scale. With high‐gradient magnetic separation (H.G.M.S.), the required process technology is available. It has already been used by various working groups in the field of bio technology. The magnetic separators used at H.G.M.S., which operate in the flow‐through mode (“magnetic filters”), were and are developed in particular by Franzreb and in own work. The advantage of this technique in the production of mR.N.A. arises from the fact that the laboratory protocols used by several users in research can be transferred 1:1 to production.10
Several novel magnetic beads are also currently in development, such as QuickGel™ beads from Quad Technologies (US), new “big beads” from CellCap Technologies Ltd (UK), and metallic beads from TurboBeads Llc (Switzerland). Many of the innovative efforts attempt to challenge the current paradigm of iron oxide beads (50nm –10μm in diameter) that remain attached to the cell surface. QuickGel™ beads are synthesized from a patented hydro-gel technology that allows for facile release of the beads from the cell surface. CellCap beads possess diameters in excess of 50μm thus their operation relies on gravitational forces combined with magnetic forces to separate labeled cells from suspension. Turbo-Beads® possess a magnetic metal core and a graphene shell of monolayer thickness; they thus present a high moment and stable labeling potential. Magnetic cell separation is a growing industry and shows much promise for continued future innovations.11 Although AC has rarely been incorporated in industrial vaccine purification, ion exchange and hydrophobic interaction chromato-graphy (H.I.C) columns have been widely used in manufacturing-scale processes in combination with other separation techniques such as filtration, precipitation and ultracentrifugation.Affinity chromato-graphy is among the most powerful separation techniques, achieving the finest separation with high yields even in the most challenging feed streams. Incorporating affinity chromato-graphy in vaccine purification has long been attempted by researchers to improve unit yield and purity with the secondary goal of reducing the number of downstream process operations. Despite the success in laboratory-scale proof of concept, implementation of this technique in pilot or cGMP manufacturing has rarely been realised due to technical and economic challenges in design and manufacturing of ideal ligands as well as availability of high-productivity chromato-graphy media. This work paper reviews evolving technologies in engineered ligands and chromato-graphy media that are encouraging companies to re-visit the possible use of affinity chromato-graphy in larger scale vaccine purification. It is postulated that commercial-scale implementation of high throughput single-use affinity chromato-graphy can significantly simplify process architecture, improve productivity and flexibility, and reduce cost of goods. Triple-helix, peptide, and amino acid-D.N.A. affinity chromato-graphy have been studied for pD.N.A. purification. R.N.A. and D.N.A. aptamers have been reported to recognize vaccinia virus, HIV, HCV, and influenza virus Figure 14.12
Rapid Methods for the Extraction of Nucleic Acids from Biological Samples
US20160333339A1
United States Google patents Patent application publication
“The invention is directed to compositions and methods for rapidly and efficiently extracting nucleic acids and/or targeted nucleic acids sequences from biological samples. The methods of the invention comprise combining the sample with a buffer and magnetic silicon beads and concentrating the beads with a magnet or other electrical field. Liquid may be removed, or not, and an alkaline buffer is added followed by magnetic carboxy beads in a binding buffer so that nucleic acids transfer to the carboxy beads, which can be easily and quickly isolated once again with a magnet. The Total nucleic -acid extraction is greatly enhanced. Extracted nucleic acids can be analyzed, for example, by PCR wherein the nucleic acids can be identified and characterized. Carboxy- beads may also contain a ligand so as to target specific nucleic acid sequences. The invention is also directed to kits comprising the tools and compositions for performing the methods of the invention
Exemplary nucleic acid capture matrix materials include, preferably, agarose, glass, cellulose, polyacrylamide, Sepharose, Sephadex, silica, or another matrix media. Preferably, the NACM material is coated with a nucleic acid binding substance (NABS), such as, for example, nucleic acid (NA) binding proteins, antibodies and chemicals with an affinity for NAs including single-stranded nucleic acid sequences. NACM include materials coupled to specific antibodies or antibody fragments or other nucleic acids or ligands that facilitate extract and/or isolation of the diagnostic molecule of interest. Affinity beads are preferably magnetic beads such as, for example, beads commercially available from Dynabeads®, Life Technologies; TurboBeads, TurboBeads Inc. or PureProteome™, and Millipore.
Micromachines Review Magnetic Bead-Magic Bullet
Christine Ruffert
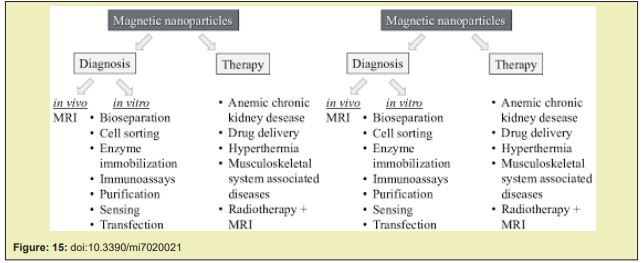
Nowadays, a huge variety of magnetic beads featuring a large diversity for different applications, is commercially available. Just to name a few: Dynabeads® Magnetic beads provided by Invitrogen,Estapor® SuperParamagnetic Microspheres and PureProteome™ Magnetic Beads by Merck Millipore, BcMag™ by Bioclone Inc., ProMag™ and BioMag® from Bangslabs, SupraMag™ by Polymicrospheres Inc., Turbo-Beads® by Turbobeads Llc., and SPHERO™ Polystyrene-Magnetic Particles by Spherotech. Other companies like Sigma-Aldrich or Thermo Scientific, Microparticles, and Microspheres-Nanospheres offer superparamagnetic beads as well. The primary use of these commercial beads is binding, purification, and magnetic separation of biomolecules comprising proteins, cells, D.N.A. fragments, and other biomolecules such as nucleic acids, enzymes, antibodies or bacteria Figure 15.13
Experimental project hypothesys
In order to verify in clear way the productive strategy in m R.N.A. vaccine manifacturing it is needed to receive from the various producers the complete documentation about the purification methods and the characteristics of the material used. The same this manifacturing process must to be verified by independent professional subjects even if regulatory GMP verify was already performed (double check). In this commision a representative of safety for patient organization must to be included to testify the oprations. Official Documents and photo must to be collected as well as the laboratory chemico analisys related. Impurity (graphene derivates in specific way) using a classic chemico anlaitycal methods whit pretreatement of the sample (solvent).14-16 It is not accettable that in official assesement report it was written that it is needed to complete the information about production of an innovative m R.N.A. COVID-19 VACCINE as well as the quality certification of the raw material used ( eccipients).17,18 Even if the MO MAYOR OBJECTION issue (GMP) was then resolved by the site of production and inspectors17 Timing of the verify : at the start of the production and every 6 month the first year , then 1 time every year. The results of this verify must to be of public availability and uploaded officially in producers website.
Related the literature reported it is clear that nowadays new methods for purifying R.N.A. are in use Versus the classic methods. Large scale production is different vs lab scale. Between various purifing method: reversde phase ion pair , anion exchange and affinity chromato-graphy is used and with magnetic beads. Ggraphene modified magnetic beads show great efficacy in this kind of process.
But because m R.N.A. VACCINE manifacturers not clarify in complete way the production process and the toxicological properties of graphene derivated are well knowed it is crucial to investigate if this new efficient technology is or not used in today production of some covid-19 vaccine. And related (19 February 2021)EMA/707383/2020 Corr.1*1 Committee for Medicinal Products for Human Use (CHMP), Assessment report Comirnaty.
Manufacturers
The active substance is manufactured and controlled by either Wyeth BioPharma Division, Andover,United States or by BioNTech Manufacturing GmbH, Mainz, Germany, and Rentschler Biopharma SE,Laupheim, Germany. During the procedure, a number of issues were highlighted relating to the GMP status of the manufacture of the active substance and of the testing sites of the finished product for the purpose of batch release. These issues were classified as a Major Objection (MO). After further information was obtained from the sites and inspectors, the MO was considered resolved. EU GMP certificates for the manufacturing and testing sites were subsequently obtained. In conclusion, appropriate manufacturing authorisations and GMP certificates are in place for all active substance and finished product manufacturing sites. 2 active substance processes have been used during the development; Process 1 and 2. The major changes between AS Process 1 and 2 are: increased process scale, D.N.A. template changed from a PCR template to linearised plasmid D.N.A., magnetic bead purification replaced with proteinase K digestionand UFDF steps. As regards SO4, the data are requested to be provided regarding the synthetic process and control strategy for the excipient ALC-0315 in order to improve the impurity control strategy, assure comprehensive quality control and batch-to-batch consistency throughout the lifecycle of the finished product
- a. A detailed description of the chemical synthesis of ALC-0315 (e.g. information on reagents and process conditions) should be provided. Due date: January 2021
- b. Differences in the manufacturing -process between two suppliers should be described and possible impact on impurity profile should be discussed by July 2021. January 2021
- c. Information and justification of quality control of starting materials (e.g. general synthetic route, supplier and specifications) and solvents should be provided. Due date: July 2021,Interim report: January 2021
- d. Information and justification on critical steps and intermediates (including specifications) should be provided. Due date: July 2021, Interim report: January 2021
- e. Specified impurities should be further evaluated and appropriate specification limits for individual impurities should be included when more data are available. Acceptance criteria for specified and un-specified impurities should be added to the specification for ALC-0315 and should also be evaluated during stability studies. Due date: July 2021, Interim report:April 2021
- f. The specification limit for total impurities should be re-evaluated as more batch data becomes available and revised, as appropriate. Due date: July 2021
- g. The specification limit for assay should be tightened based on the provided batch data to improve the quality control strategy of the finished product. Due date: July 2021
- h. Detailed method validation reports for assay, impurities, and residual solvents for ALC-0315 should be provided. Due date: July 2021
- i. Results of stability studies in accordance with ICH guidelines should be provided. Due date: July 2021, Interim report: April 2021 “
It is clear that today m R.N.A. Vaccine manifacturing process use non classic methods in purification phases. Between the more recent technology are also used affinity chromatograpy and high‐gradient magnetic separation (H.G.M.S.). Magnetic beads are used in this process. Magnetic beads are produced bay various industry using different technology13 Some producers provides also graphene modified magnetic beads to increase efficiency of the process. For this reason it is necessary that the vaccine producers provide a complete and full information about the complete manifacturing process as well as the methodology used in purification. All this related the various techologies in use, the need to get great efficiency in productions and the toxicological implications if impurity of graphene is finded (or not ) in final products of vaccine. In natural way it is possible that producers like to use the really best efficient techinologies to get better results in manifacturing but this must to be linked to the toxicological limits if dangerous substantie are used in the purification process.
None.
None.
Authors declare that there are no conflicts of interest.
- 1. Cheng Xu, Hao Hong, Yonghyun Lee, et al. Efficient lymph node-targeted delivery of personalized cancer vaccines with reactive oxygen species-inducing reduced graphene oxide nanosheets. ACS Nano. 2020;14(10):13268–13278.
- 2. Yue Yin, Xiaoyang Li, Haixia Ma, et al. In situ transforming r.n.a. nanovaccines from polyethylenimine functionalized graphene oxide hydro-gel for durable cancer immunotherapy. Nano Lett. 2021;21(5):2224–2231.
- 3. Yunfei Wang, Guoping Sun, Yingying Gong, et al. Functionalized folate-modified graphene oxide/pei sir.n.a. nanocomplexes for targeted ovarian cancer gene therapy. Nano Express Open Access Published: 06 March 2020.
- 4. S Garaj, W Hubbard, A Reina, et al. Graphene as a subnanometre trans-electrode membrane. Nature volume. 2010;Pp.190–193.
- 5. S Syama, PV Mohanan. Comprehensive application of graphene: emphasis on biomedical concerns. Nano-Micro Letters. 219(11).
- 6. Hye Yeon Choi, Tae Jin Lee, Gwang Mo Yang, et al. Efficient mR.N.A. delivery with graphene oxide-polyethylenimine for generation of footprint-free human induced pluripotent stem cells. J Control Release. 2016;(10):222–235.
- 7. Young ro. Scanning and transmission electron microscopy reveals graphene oxide in CoV-19 vaccines. Materials Science Acta Scientific Medical Sciences. 2022: 237952830.
- 8. CNIPAChina national intellectual property administration Nano coronavirus recombinant vaccine taking graphene oxide as carrier.
- 9. Xuan Hung Pham, Ahruem Baek, Tae Han Kim, et al. Graphene Oxide Conjugated Magnetic Beads for R.N.A. Extraction. Chem Asian J . 2017;12(15):1883–1888.
- 10. Lars Wommer, Patrick Meiers, Isabelle Kockler, et al. Development of a 3D‐printed single‐use separation chamber for use in mR.N.A.‐based vaccine production with magnetic microparticles. Eng Life Sci. 2021;21(10):573–588.
- 11. Brian D Plouffe, Shashi K Murthy, Laura H Lewis. Fundamentals and Application of Magnetic Particles in Cell Isolation and Enrichment. Rep Prog Phys. 2015;78(1):016601.
- 12. Mochao Zhao, Melissa Vandersluis, James Stout, et al. Affinity chromato-graphy for vaccines manufacturing: Finally ready for prime time?. Vaccine. 2018;;37(36):5491–5503.
- 13. Stephanus Büttgenbach. Center for Production Technology, Leibniz Universitaet Hannover, An der Universitaet 2, D-30823 Garbsen, micromachines Review Magnetic Bead-Magic Bullet Christine Ruffert.
- 14. Luisetto M , Almukthar N, Tarro , et al. Graphene and Derivates: Physico-Chemical and Toxicology Properties in the mR.N.A. Vaccine Manifacturing Strategy. Sci World J Pharm Sci. 2022;1(2):1–23.
- 15. Biopharmaceutical large scale production:The graphene - derivates role" Lambert academic publishing sept 2022
- 16. Luisetto M, Nili B, Edbey K, et al. Raman (Rs) Spectroscopy for Biopharmaceutical Quality Control and PAT. Raw Material - Final Products: the Nanolipids Effect on Signal Intensity. Regulatory and Toxicological Aspects. Med & Analy Chem Int J. 2022;6(1).
- 17. Assessment Report EMA/Pfizer Comirnaty, 19 feb. 2021
- 18. AIFA Autorization drug of new registration conditioned . Rep. n. 154/2020 del 23/12/2020.

