Background: Anaesthesia and surgical interventions cause significant changes in body temperature and glycaemia control in human and in animals. Different measures were successfully applied to counter these changes. On the other hand, the treatment of acute or chronic glucose metabolism disorders based only on the evolution of serum glucose is not entirely satisfactory, using a device (ADD/) to measure the evolution of core/deep and surface body temperatures and their difference (∆t), we investigated the relation between ∆t and glycaemia during anaesthesia in healthy and diabetic animals (rats) and during surgical operations in humans. Therefore, we followed the spontaneous evolution of glycaemia and body temperatures during anaesthesia and surgical stress, with or without interventions (insulin and glucose perfusion) to verify/justify the possibility of the intervention monitoring following ∆t evolution.
Methods: Fifty two Wistar rats were used, 26 as controls and 26 with an experimental diabetes induced with streptozotocin to investigate the effect of anaesthesia and surgical stress alone. Another group of 26 Wistar rats were used in the same conditions plus insulin and glucose intravenous injection.
The experiments were conducted in standard conditions of room temperature. After anaesthetics administration glycaemia was measured every 15-30min. Deep and superficial temperatures and their difference ΔT were registered continuously using an ADD device.
Results: In intact anaesthetized animals after a slight elevation during the first 30 minutes, glycaemia decreased regularly with time during anaesthesia body temperature gradient (∆t) absolute values depended on the position of the temperature sensors and on ambient temperature, but their evolution was the same: slight initial decrease, stabilization and slight elevation before waking. Correlation between the two parameters was not evident. Thoracic surgery caused a more pronounced temperature decrease and glycaemia changes than anaesthesia alone (∆t not measured). In diabetic animals, as a rule glycaemia remained high during the operation time, the variations of ∆t values were more important and prolonged, as a rule ∆t was lower in diabetic animals than in healthy ones. Correlation with glycaemia could not be detected. Comparison between investigation results in animals before and after diabetes induction has pointed the important differences due to the disease and confirmed that ∆t reactions always preceded glycaemia ones) that explain the absence of correlation between these parameters). In all the series anaesthetic overdose could cause a temporary negative ∆t even in presence of normo- or hyperglycaemia. Ambient temperature elevation >30°C during the sessions caused an increase of all investigated features absolute values but none of their evolution. Taking into account the quick reaction of ∆t to modifications of external and internal conditions, monitoring glycaemia disorders by balanced insulin and glucose intravenous injection guided by ∆t evolution was tried with positive encouraging results. Clinical observations were added which results were close to the experimental ones, as well when concerning the influence of external (temperature) and internal (anaesthesia), metabolic factors, as when confirm possibility of monitoring insulin administration with energetic feedback.
Conclusion: This study confirms that stress, ambient temperature and anaesthesia can alter both glycaemia and body temperature evolution, and that more profoundly in diabetes. It has shown a high sensibility of ∆t to the metabolic changes due to these factors. It ought to allow a valuable algorithm elaboration for glucose and insulin administration in automatic monitoring of energetic balance by a new ADD-CIGT device.
Keywords: Anaesthesia, Artificial pancreas, Body temperatures, Diabetes, Energetic balance, Glucose metabolism, Glycaemia regulation, Insulin pump
Abbreviations: ADD: Apparatus for Diabetes Diagnosis; ADD-CIT: Apparatus for Diabetes Diagnosis with Complex Insulin Therapy added; BW: Body weight; DWV: Drunk water volume; M: Mean value; SD: Standard deviation; ∆t: Difference between Tc and Ts Tc-Core or deep temperature; Ts: Superficial temperature; THI: Thermometry investigation
Glycaemia can be altered in many situations: all forms of diabetes mellitus (DM), acute critical syndromes (shocks, sepsis and others including COVID), surgical interventions.1-5 The classical treatment of hyperglycaemia is insulin administration according to standard protocols and using well known devices, always based on glycaemia measure as a feedback attesting the procedure efficacy,6-9 but a satisfactory control of glycaemia can be difficult to reach.6-13 Since some decades a new feedback based on measures of body temperatures evolution was proposed14-15 and has shown some advantages in acute life threatening situations, whatever the origin of the hyperglycaemia.16-21 However, no correlation was found between recorded glycaemia and temperature evolutions in spite of the fact that the glucose metabolism generates 70% of the body energy.22 The link between glycaemia and body temperatures has been weakly investigated. It is well known that in human a temperature fall (besides hyperglycaemia tendency) occurs during surgical interventions.23-26 and warming the patient by different means (matrass, cover, and so on) is current in operating rooms. But, to our knowledge, systematic investigations of the evolution of the body temperature during surgery have not been performed. On the contrary, glycaemia is measured currently and insulin shots are given according to standard protocols. In animals, anaesthesia and operation stress were shown to cause serious body temperature and glycaemia disorders, both developing without evident correlation.27 The reaction to external temperature changes (global heat or cold, local cooling or warming) differs in diabetics and healthy subjects.28,29 Such a difference is also observed in animals.30
The aim of our work was to assess the evolution of the body temperature and of glycaemia in normal and streptozotocin induced diabetic rats submitted to surgical stress and anaesthesia, with the following objectives:
- i. To point the difference in reactions of healthy and DM animals to stress and anaesthesia,
- ii. To determine the specificity of the investigated factors (stress, anaesthesia and STZ induced glucose disorders) on glycaemia and body temperatures,
- iii. To verify whether a “normal” pattern of body temperature evolution may be observed,
- iv. If it could be a model for glycaemia regulation in pathological situations and explain the success of the ADD-CIT use in clinics and offer a basis for elaboration of CIGT device.
Experimentation in rats
The experiments were conducted on 112 Wistar adult rats displayed in 3 large groups: 41 animals (22 males, 19 females) for observational (part 1), 27 animals (12 males, 15 females) for interventional (part 2), and 34 animals for control investigations (paired and during thoracic surgery (Table 1)). All were aged 9.7±4.2 (6-24) months. It is to be noted that after 6 month age, BW curve in males and females smooths and relatively stabilizes (see Catalogues of the animal trade). All the manipulations on animals and ADD sessions were performed under Anaesthesia according to the following protocol: induction with Fluorotane 4%, 1min/100g BW, or use of flexible/supple/soft contention device.31 which seems to diminish stress, and main intra peritoneal bolus injection of Pentobarbital Natrium (0.075mg/100gBW) diluted 1:10 in saline. After 2019, Pentobarbital was replaced by intra peritoneal bolus injection of Ketalar (0.1ml/100gBW), Diazepam (0.1ml 1% solution,) and Temgesic (0.1ml/100gBW). For thoracic surgery, a subcutaneous injection of Atropine sulphate 1% and Temgesic 1% were added (both 0.2ml).
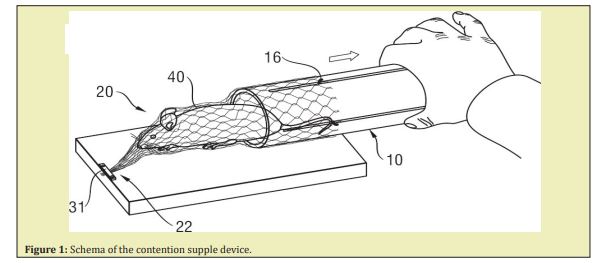
At the end of the investigation the animals were euthanatized by injection of a lethal dose of anaesthetic. To test the individual characteristic influence on the main investigated data, paired experiences were provided, when the same animal was used successively for determination of blood glucose under anaesthesia without thermometry (TH), the next day - with thermometry, and at last after STZ injection (day 2,5±1). The majority of the experiments were conducted at ambient temperature (24-26°C) under a warming lamp. For thoracic surgery, the animal was placed on a warming support with 39°C permanent controlled temperature. As a short time increase of the ambient temperature is known to cause a temporary increase of deep body elevated ambient temperature on body temperatures, some experiments were conducted at higher ambient temperature of 30-31°C. To determine the impact of the localization of the temperature captors/sensors within the rectum and knowing that the distance between them is 3cm (to avoid interferences), the catheter was placed in two positions: with the superficial sensor at 3cm or at 2cm from the anal marge (3 paired séances). For exploration of major surgery influence the model of heart lesion with or without repair was used and described elsewhere.32 For insulin/glucose treatment, insulin Aktrapid 20UI/, and glucose 30%ml were used. For intravenous injection a BED Neoflon Bluer Lok26G0 6x19mm catheter was inserted and fixed into the jugular vein of the animal.
The following investigations were provided:
- a. BW measure-daily
- b. Drunk water volume (DWV) determination – daily
- c. Glycaemia measure by strip method (One Touch- Verio IQ, LifeScan, Switzerland). Blood for glucose determination was collected from the tail vessels 2 minutes after complete anaesthesia (that is 5-10 minutes after anaesthetics administration and after catheter insertion - once every 15-30min, till animal wakening.
The animals were managed according to the Bioethics rules of animal welfare (Helsinki Convention 1964, Belgian Official documents 2010, 2013, and the local Ethics Committee (protocols n°480, 508 and 735). Clinical investigations were also allowed by the Ethic Commission of the CHU Brugmann (2002/35; 2017/66).
Observations in humans
In the clinic 5 informed volunteers were tested without anaesthesia and 16 consenting informed patients were investigated during severe high abdominal surgery, performed under usual classic anaesthesia using Propofol or Etomidate or Ketalar as inductors and Sufentanyl for the main analgesia. Glycaemia was measured, every hour either by strips (Acutrends-USA, One Touch- Verio IQ, LifeScan, Switzerland) or by pH meter. Body temperature was measured by device ADD8 in continuity, beginning within the first 10-15min after anaesthetics administration.
*For remaining ADD device consists in catheter containing 2 temperature sensors placed at a 3cm distance (for use in the rat), 408cm for humans, and an analyser with a screen showing Td, Ts and ∆t evolution, (Figure 1).
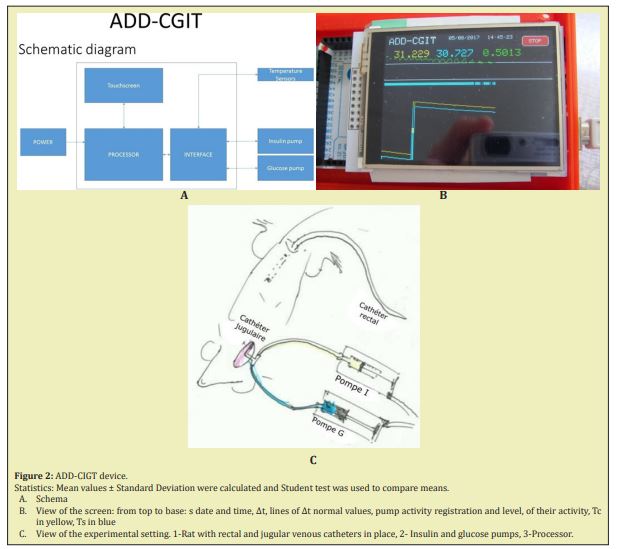
*The ADD device in its last modification was composed of a double temperature digital sensor for body deep and superficial temperature registration, Atmel AVR processor, TFT touch screen and injection pumps for insulin and glucose automated injection were used, a processor and an interface for communication with insulin and glucose pumps (Figure 2). The sensors were included at a 4.5cm distance from each other, 3cm for rats (to avoid temperature influence on each other) into an isolated catheter of 4mm diameter enclosed into a thin, waterproof and one-using polyethylene membrane. When the catheter was placed in the rectum the inner sensor at the top of the catheter was situated at the level of the deep haemorrhoidal venous plexus in human, at the hepatic angle of the rat colon, and registered the core or deep temperature (Tc). The lower sensor was placed just above the external anal sphincter and registered the superficial temperature (Ts) of the subcutaneous para-rectal fat. In this study the device was used only in observation regimen, i.e. for temperature registering. Step pumps for insulin and glucose injection commanded through the device but manually regulated were added. Some trials to use insulin and glucose pumps for influencing temperature fall and glycaemia were provided in 27 rats (intact or diabetic). In all the cases (except healthy humans) the rectal catheter was introduced after sedation or anaesthesia (Figure 2).
Part one. Healthy animals
Animals characteristics: In the different series, BW was relatively stable and comparable in the different series and groups in males as in females (Table 3).
Effect of ambient temperature on TC, TS and Delta T: Testing different ambient temperatures has shown a slight increase of the Tc and TS level, no modification of ∆t and no significant modification of the general evolution of the parameters Nevertheless, ambient temperature 24-26°C was preferred (Figure 4).
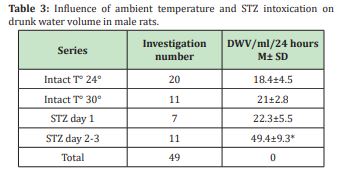
Effect of anaesthesia alone: In the series when anaesthetics administration was the only influencing factor a slight not significant increase of glycaemia was noted 15min after the anaesthetics injection. Later a progressive but not significant decrease was observed till the last minutes before wakening. An important variability of the initial values was noted but it decreased with observation time (20% at time 0 versus 8% from time 4) (Figure 3). In the series with temperature registration (THI), a second not significant elevation of glycaemia was noted after the first 15 and 45min. A significant increasing preceded the wakening (mini 20-150 versus min 180 (Figure 4A).
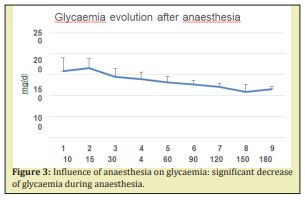
Influence of BW: As Figure 4 shows, the difference in BW between males and females did not seem to modify neither the glycaemia nor the body temperature evolution during a three hour observation In the different series, the sex of animals had an influence on the tolerance of anaesthesia: in females 50-100% increase of the anaesthesia duration (for the same dose 100gBW) was noted, in males wakening occurred after 80-90min (N=20), females after 180min (N=21). Concerning the glycaemia or temperature values the same tendencies were observed in both sexes i.e. a decrease during the anaesthesia proportional to its duration and an increase at the wakening (Figure 4).
Stress caused by introduction of the rectal catheter: The stress caused by the catheter introduction into the rectum after anaesthesia was practically absent and did not significantly affect the glycaemia though in some animals 2 distinct elevation pikes of glycaemia could be noted after 15 related to anaesthesia induction and after 30-45min due to catheter introduction.
Influence of ambient temperature: Ambient temperature levels (25°C versus 30°C) had no significant influence on the daily drunk water volume (DWV) which varied from 9 to 25ml (M ± SD: 18.4±4.5ml) at 25°C and between 12 and 29ml (21±2.8ml) when temperature increased up to 30°C. Unless it remained constant during the investigation duration, ambient temperature levels have not caused any significant changes in measured glycaemia levels and body temperature evolution. A slight difference was noted in the initial core and superficial temperatures but not on their difference ∆t or their evolution (Figure 4B–4D).
Localisation of the superficial sensor: Localization of the superficial temperature sensor was shown influencing the observed superficial body temperatures and so ∆t. In 5 animals the superficial sensor was in the rectum at 2cm from the anal marge instead of 3cm. In this group Tc remains the same, Ts was about 0.5°C lower in the first 30min so ∆t was higher (Figure 4E–4G). The evolution of the 3 parameters was globally the same as in the other animals according to the calculated percentage of the values relatively to the initial one. Nevertheless, the position for the catheter with a Ts sensor situated at 3cm of the anal marge was preferred and adopted (then Tc sensor at 6cm).
Impact of thoracic surgery: In control series with thoracic surgery the unique sensor placed into the rectum at about 2,5cm from the anal marge has shown the same tendency of progressive fall from initial 36°C to 32°C and even lower. This was observed in spite of a warming surface of 39°C under the spine of the animal. The temperature increasing was a positive prognostic event announcing a quick waking. Irreversible fall of temperature under the 30°C was a sure prediction of lethal issue.
Part 2. Studies in animals with STZ induced diabetes
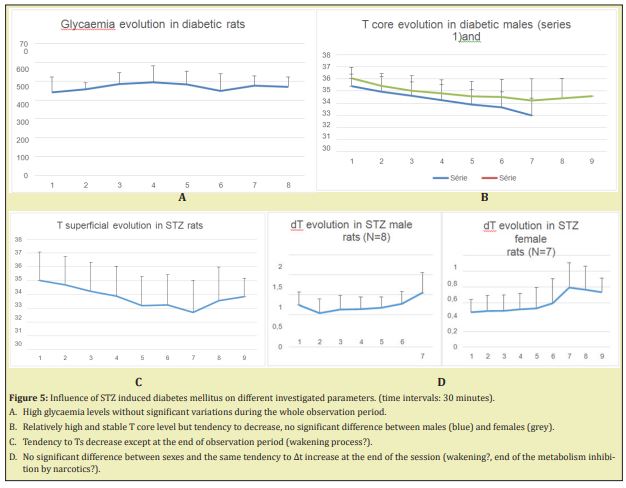
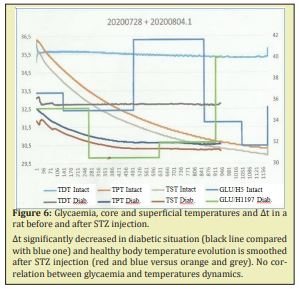
STZ diabetes without treatment: After injection of STZ, DWV significantly increased and reached up to 73ml/day at day 3-4. At day 1 the increasing was not yet significant (22.3±5.5ml, N volume in male rats) (Table 3). So the preferred moment for glycaemia investigation was day 2 and further. In diabetic rats glycaemia remained high and stable during anaesthesia (Figure 5A). As far as temperature parameters were concerned, a large variability was observed (up to 35% for ∆t). Core and superficial temperatures decreased with time under anaesthesia, but ∆t remained at the same level or increased at the end of the observation (Figure 5B-5D). The comparison of the data’s obtained in healthy and in diabetic rats has shown a clear and significant difference in glycaemia levels (p<0.001) but temperature evolutions alterations, though present, were less evident. In spite of maximal standardization of the conditions of the experiences, a high variability of the parameters from the beginning of the observation, that could smooth the statistics significance of the tendencies noted between the values of all the investigated parameters either during the observation or in different groups, even in diabetic rats with high glycaemia. Investigation of the same animal before and after diabetes induction (paired experiments) could minimize or exclude the influence of individual characteristics on the statistics. Paired cases have shown that in 9 cases out of 10, diabetes development has led not only to glycaemia drastic increase, but also to serious temperature disorders, particularly in ∆t, which was systematically less after diabetes induction. The evolution of Tc and Ts was also different: the decrease slope was much more expressed in healthy animals, than in diabetic ones (Figure 6). Interesting to note that the only animal showing an exception to this rule, occurred to have been tolerant to STZ administration. ∆t significantly decreased in diabetic situation (black line compared with blue one) and healthy body temperature evolution is smoothed after STZ injection (red and blue versus orange and grey). No correlation between glycaemia and temperatures dynamics. These experiments have also confirm that ∆t modifications could be observed 30-40min before the start of glycaemia ones.
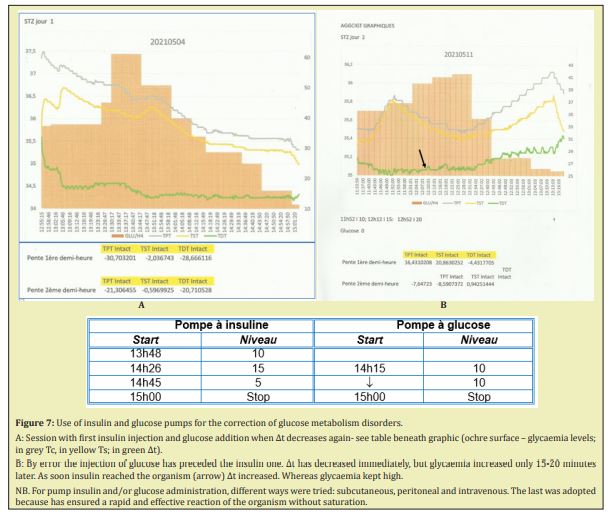
STZ diabetes with monitoring of insulin and glucose administration.
We tried to correct hyperglycaemia by pump injections of insulin and glucose, monitoring the levels of injection, not according to glycaemia but following the ∆t variations. As shown on the Figure 7 insulin administration at a high level (20 impulses/min) has allowed an early stabilization of ∆t and 30 min later, the beginning of glycaemia decrease. A new decrease of ∆t has conditioned the beginning of glucose injection (10 impulses/min), followed by stabilization of ∆t and the continuation of regular, smooth glycaemia decrease (Figure 7A). In one case the case of misadministration of glucose, which has caused both increase of glycaemia (delayed for 35min) but immediate fall of ∆t Arrival of insulin into the injection catheter has corrected the glycaemia but altered the ∆t. Adjustment of insulin and glucose administration has allowed to stabilize ∆t and ensured a smooth decrease of glycaemia, the pump regimens being manually monitored according to the ∆t evolution (Figure 7B).
Part three. Investigation of Humans
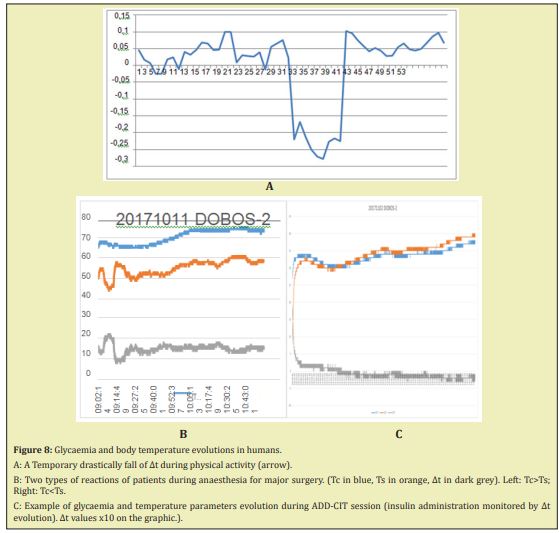
In Human healthy adults at rest without sedation or anaesthesia glycaemia remained in the normal range of 70-90mg/dl during the whole observation period, slightly decreasing after 2 hours if no food was given. ∆t values were between 0.05 and 0.15°C. Introduction of significant physical activity caused a quasi-immediate significant fall of ∆t up to negative values with rapid restoration as soon as activity stopped (Figure 8A), whereas, besides, glycaemia was not affected. ∆t increased after food intake.
During anaesthesia for major abdominal surgery, glycaemia and temperature registration have shown in all 17 cases a tendency to smooth decrease followed by a normalization of the temperature at the initial level. The maximal body temperature loss represented a 1-2% in comparison with the initial temperature. An increase of blood glucose level was also noted, that justify in some cases (mainly diabetic or pre diabetic patients) insulin injection. ∆t observed was positive in 2/3 of the cases but negative in the last third. The links between this difference and the pathology having necessitated the surgery, co morbidities, age or other causes were not found. The use in clinics of ∆t feedback for insulin administration monitoring in critical acute glucose metabolism disorders in diabetic and not diabetic patients with heart, kidney pathologies, after pancreas and/or kidney transplantation, has been reported elsewhere.16-21 The summarized results showed a successful use of ADD in more than 70% of the cases. Repeated sessions were sometimes performed to improve results. Nevertheless, it is necessary to note that in many cases, at the end of the ADD-CIT session, a glucose 20% shot injection was necessary to prevent hypoglycaemia development. During the sessions some cases of hypoglycaemia were registered, but they were less frequent than with classical treatment.
Before starting discussion, limitations of the study are to be considered:
- Absence of continuous blood glucose registration, but only repeated control measures of glycaemia at 10-30 minutes interval in animals, once an hour in clinics. It is to be noted that glycaemia measure in time interval time range of 1-5min have shown variations of the results within the limits of the method error. In the experimental series glycaemia was controlled no less than every 15-20min. In human investigations, glycaemia control was realized once an hour.
- The experiments were conducted at different moments of the year but the season variations of atmosphere pressure, luminosity, hygrometric and other climatic conditions were not evaluated but could affect the results.
- Ambient temperature and location of the catheter, conditioning mainly Ts, were not strictly observed though optimal ciphers were found and respected in different series.
- Investigation conditions in humans were different of the experimental ones that has to be taken into account making difficult some comparisons.
The analysis of results of diriment series has pointed several moments. Experiments have not shown any significant influence of age, body weight on the studied parameters, probably because adult animals were chosen, when BW remains relatively stable even in males. In all the series, the sex of animals was determinant only for a 50-100% prolongation of the anaesthetics effect for the same dose /100gBW in females: in all series males awaked after 80-90min, females after 150-180min. This corresponded to a tendency of Tc and Ts to decrease during a longer period of time in females than in males (Figures 4,5). Nevertheless, variability of glycaemia, temperature and especially of ∆t initial values points to individual reactions to the environment (external and internal). DWV increase has helped to identify the STZ influence as well as glycaemia increase. Ambient temperature modifications (25°C versus 30°C) had some influence unless ambient temperature remained constant during the investigation duration, no significant changes in measured glycaemia levels were observed. The same concerned body temperatures evolution. Only a slight increase of the initial core and superficial temperature as the result of thermogenesis/metabolic activity boosting s was noted but not of their difference ∆t or their evolution. Maintaining ambient temperature of 25±1°C adopted in the majority of the experiences allowed a standardization of the experimental conditions and facilitated their result analysis. In the experiments with heart surgery matrass warming did not prevent severe decrease of body temperature, not only because the thoracic cavity was opened, but also probably because the body metabolism was deeply decreased due both to anaesthesia and surgery. Localization of the captor of superficial temperature was shown to influence the Ts temperatures and ∆t, while in any case, the Tc captor was in the core zone. Besides, the general trends of the 3 temperature parameters evolution remained the same unless the position of the catheter was constant during the observation time (Figures 4,5). So this factor could be neglected, as the previous ones. So, as far as the conditions of the experiment were stable, such as ambient temperature, stress neutralized by analgesia and sedation (this is the case in clinics), the influence of anaesthesia could be considered as predominant. They were practically the same in the control intact animal series and caused a progressive decrease of glycaemia (up to subnormal values). A decrease of Tc, and Ts down to 32°C, in some cases, stopped only by the wakening beginning moment (Figure 3). This is in keeping with previous observations23-27 and is probably due to the inhibition of vital activity including carbohydrate metabolism due to the anaesthetics drugs. It may be interpreted as a decrease of thermogenesis accompanying a decrease of energetic loss. ∆t was more or less stable because the Tc and Ts decrease was in general proportional, except in the cases when energetic reserve was very low (glycaemia about zero). In that case, Ts increased and ∆t showed regular oscillations greater 0.1°C. Anaesthetics overdoses always ended by drastic and irreversible hypoglycaemia and temperature decrease with inversion of ∆t sign, with Ts increasing probably reflecting the mobilization of pentose metabolism in paravertebral caudal brown fat sustaining thermogenesis by lipolysis.34-36 In diabetic rats without treatment, this was not observed: hyper glycaemia was stable even while body temperatures and ∆t were low and varied (Figures 4–6). This may confirm the presence of severe metabolic disorders detected both by physical and biochemical methods. It also suggests that temperature evolution might be more sensitive and as liable, than glycaemia registration and so usefully complete it. Besides, stress influence was minimized and weak only when anaesthesia was adequate. A mild glycaemia elevation could be observed (delayed); a ∆t and TC, TS increasing might be a useful sign of wakening.
In clinical conditions, the success of anaesthesia could be assessed by a smooth parameter evolution curve, and parameters values close to normal ones as observed in persons who were not under anaesthesia. Detection of negative ∆t may be an interesting prognostic sign, not understood yet. It is possible that absolute values of ∆t are less important than their evolution (see data from ADD-CIT session). This could explain the rare cases when negative ∆t were noted at the end of monitored insulin administration with glycaemia normalization. In fact, keeping body temperature and ∆t stable by different therapeutic actions (perfusion, oxygenation, analgesia, and others), the anaesthetists ensure a satisfying basal metabolism and thus the favourable surgery issue. Consequently in the same conditions, observed alterations of the parameters values and evolution could be considered as specific consequences of diabetes mellitus. In rats, STZ induced diabetes might explain the differences of the glycaemia and body temperature gradients compared to control (healthy animals) under the same anaesthesia schedule. These differences were clearly demonstrated in paired experiments when Tc and Ts evolution was smoothed and ∆t - lower in comparison with control (Figure 6). The influence of diabetes on reactions to temperature modifications was described in literature,29-31 but on the contrary – temperature modifications due to diabetes seems not having been considered. Besides, ∆t levels could be a diagnostic test for pre and florid diabetes.15,16
If so, correction of glycaemia must be accompanied and even preceded by body temperature changes. The series with insulin administration, in rats as in men, have confirmed this hypothesis.16-21,32 It was noted and confirmed by other authors, that glycaemia improvement was significantly delayed considering the metabolic event. This was also considered as a cause of insulin classic therapy difficulties and some trials to overcome the difficulty were proposed.10,11 Glycaemia feedback remains the unique proposal for insulin therapy monitoring up to now. Nevertheless, in spite of encouraging results including a decrease of hypoglycaemia and yo-yo phenomena events, the last were still observed, especially at the end of some ADD-CIT sessions that required glucose shot injections. This has incited us to consider and try the possibility of including a glucose administration automatically monitored by ∆t feedback. Our trial of manual monitoring the glucose administration by a pump regulated according to ∆t variations, seems promising and further investigation and development would be worthwhile, including automation, distant temperature registration, application to chronic long time use and so on. Besides the success of the attempt was linked with an intravenous drug administration, which allow a rapid effect of the injected substances. The choice of concentration is also important to ensure an optimal effect without, in the rat, a minimal liquid inflow (about 1 ml/hour). So ∆t and approach of algorithm elaboration for insulin and glucose pumps automation seems worthwhile further investigations.
This study allows to confirm the specificity of anaesthesia and diabetes influence on glycaemia and body temperatures. Delayed reaction of glycaemia to insulin and even glucose injections was proven and explained. The glycaemia is the result of different influences (outcome – physical exercises, digestion processes, and so on, income, alimentation, hormonal processes, and so on), so its modification according to conditions changes need some time to develop The ∆t evolution (∆t slope) reflects the energetic combustion process which is immediately affected by metabolic reactions The hypothesis of a strong link between ∆t evolution and energetic/glucose metabolism seems to confirm. Possible use of ∆t evolution as a feedback for glucose metabolism disorders treatment seems more and more valuable. So, glycaemia as well as ∆t evolution seems to reflect the energetic metabolism, but glycaemia represents the coal not yet used and remaining in the coal box, whereas ∆t reflects the combustion process itself. Hence ∆t could be a more sensible instrument for monitoring glucose/energetic metabolism correction in acute and possibly in chronic disorders.
To Drs A Bodson and G Krzentowski (CHU Marie Curie) Me JM Menne, who supported and encouraged the work from its very beginning; To Professor P Bergmann (ULB) for his precious help in precise formulation of the concepts, experimental design and results interpretation, and Dr P Delrée for the comprehension and precious help; To Mrs M Leroy for logistic help; To Mr A Bekkouri, Mr R Creton and the other volunteers who have contributed to the study; To Mr Ingrassa and his colleagues of the ULB TTO who have tried to help and support the study; To Mrs Y. Messe Coulic who was sceptic but always bravely has supported the study.
None.
Authors declare that there are no conflicts of interest.
- 1. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367.
- 2. Van den Berghe G, Wilmer G, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461.
- 3. Van den Berghe G, Wilmer G, Milants I, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: good versus harm. Diabetes. 2006; 55(11):3151–3159.
- 4. Thomann R, Keller U. “Hyperglycémie dans les maladies aiguës-un risqué sucré”. Forum médical Suisse. 2006;6:1051–1054.
- 5. Fahy BG, Sheehy AM, Cousin DB. Glucose control in the intensive care unit. Crit Care Med. 2009;37(5):1769–1776.
- 6. Hirsch B. Understanding low sugar from NICE-SUGAR. N Engl J Med. 2012;367(12):1150–1152.
- 7. Kavanagh BP. Glucose in the ICU-Evidences, guidelines and outcomes. N Engl J Med. 2012;367(13):1259–1260.
- 8. José Panza Nduli, Very Coulic, Dominique Willems, Jacques Devriendt et al. Influence of bedside insulin measurement on acute coronary syndrome pathways. Critic Pathways Cardiology. 2011;10(4):185–188.
- 9. Lazzari C, Bonizzoli M, Blacchi S, et al. The pronostic role of Hyperglycemia and glucose variability in covid-related acute respiratory distress syndrome. Diabetes research and clinical, practice. 2021;175:108789.
- 10. Harvey RA, Dassau E, Zisser H, et al. Design of the health monitoring systeetes Sci Technol. 2012;6(6):1345–1354.
- 11. Facchinetti A, Sparacino G, Guerra S, et al. Real-time improvement of continuous glucose-monitoring accuracy: the smart sensor concept. Diabetes Care. 2012;36(4):793–800.
- 12. Sparacino G, Zanon M, Facchinetti A, et al. Italian contribution to the development of continuous glucose monitoring sensors for diabetes management. Sensors. 2012;12(10):13753–13780.
- 13. Davidson P, Steed RD, Bode BW. Glucpmander. A computer-directed intravenous insulin system shown to be safe, simple and effectrive in 120,618h of operation. Diabetes Care. 2005;28(10):2418–2423.
- 14. Novikov VK, Coulic VP “Méthode/Procédé de diagnostic du diabète sucré” Moscou 15.11.1991 Brevet n° 1718822. (priorité 1987).
- 15. Somonov VA, Kluchko AV, Novikov VK, et al. Electronic device for control of energetic balanve in man ancd animal (appliance in Diabetes Mellitus). Conf Procceddins IEEEng Biol Med Soc. 1992;6:2270–2271.
- 16. Coulic Véry, Novikov Valéri, Devriendt Jacques, et al. Use of temperature gradient measuring device in monitoring of diabetic and critically ill patients.” Annu Int Conf IEEE Eng Med Biol Soc. 2007;372.
- 17. Tarabarko NV, Novikov VK, Rjevskaya ON, et al. Surgical treatment of Diabetes Mellitus. Viestnik Transplantologyi y Iskustvennykh Organov. 2006;8(4):23–30.
- 18. Tarabarko NV, Novikov VK, Rjevskaya ON, et al. Combined transplantation of a kidney and pancreatoduodenal complex in the treatment of Diabetes Mellitus. Viestnik Transplantologyi y Iskustvennykh Organov. 2007;9(5):8–14.
- 19. Novikov VK, Vietluguina MA, Maïssiouk IaG. Correction of carbohydrate metabolism by using the apparatus of insulin therapy in patients with type I Diabetes Mellitus after renal transplantation. Viestnik Transplantologyi y Iskustvennykh Organov. 2012;14:72–76.
- 20. Novikov V, Anissimov Iu, Dmitriev I, et al. Differential Temperature Evolution for Insulin Delivery Monitoring in Type 1 Diabetic Patients before and after Kidney-Pancreas Transplantation. IJDCD. 2016;2394–1499.
- 21. Novikov VK, Dobos S, Devriendt J, et al. Is the new “energetic” feedback for insulin delivery monitoring available in any acute severe glucose metabolism disorders? EMIJ. 2018;20(6):318–322.
- 22. Schmit RF, Tes G. Human Physiology. Moscow: Nauka. 1986.
- 23. Grote R, Wetz AJ, Bräuer A, et al. Prewarning according to the AWMF S3 guidelines on preventing inadvertent perioperative hypothermia. Anaesthesist. 2017;67(1):27–33.
- 24. Monteiro FLJ, Halpern H, Bortoli F, et al. Forced air-warming in patients undergoing endovascular procedures. Comparison between 2 theermal blanket models. Ann Vasc Surg. 2018;47:98–103.
- 25. Schuster CJ, Pang DSJ. Forced air pre-warming prevents peri-anaesthetic hypothermia and shorters recovery in adult rats. Lab Anim. 2018;52(2):142–151.
- 26. Epstein FH, Dexter F, Hofer IS, et al. Perioperative temperature measurement consideration relevant to reporting requirements for National Quality Programs using data from Anaesthesia information management system. Anesth Analg. 2018;126(2):478–486.
- 27. Coulic V, Martin M, Dobos S, et al. Anaesthesia influence on glycaemia and body temperature (First results). Endocrinology & Metabolism International Journal. 2017;6(2):00154.
- 28. Kitamura H, Hoshino T, Kon T, et al. Patients with diabetic neuropathy are at risk of greater intra operative reduction in core temperature. Aneshesiology. 2000;92:131–138.
- 29. Fujiwara Y, Inukai T, Aso Y, et al. Thermographic measurement of skun temperaturerecovery time of extremities in patients with type 2 diabetes mellitus. Exper Clon Endocrinol diabetes. 2000;108:463–469.
- 30. NBovikov VK, Kulik VP. Effect of Streptozotocin on temperature varuations in rats. BillExper Biol and Med. 1990;110:36362.
- 31. Novikov VK, Dewrée R, Coulic V. Method of restraining an animal and device therefor. Moyen de contention souple élastique non traumatique; BREVET WO. 2013;185817 A17.
- 32. Quéron S, Coulic V, Stefanidis K, Delrée P, DePrez C, Najar Saligheh E, Bergmann P. A new (Alternative) Model of heart Lesion in Rats: A Step to Cardiomyoblast Grafting for Cardiac Tissue Repair. Medicine and Medical Research. 2020;4:16–31.
- 33. Novikov VK, Coulic VP. Moyen de normalisation du métabolisme des glucides incluant la détermination du niveau de glycémie et l’injection d’insuline en quantité suffisante pour atteindre une glycémie normale, se distinguant par le fait que, au lieu de déterminer le niveau de la glycémie, la différence de température entre le noyau thermique central du corps et la température de la couche adipeuse sous-cutanée est déterminée et on injecte l’insuline en quantité suffisante pour atteindre un différence de températures de à0.15°C. 2011.
- 34. Kabta Chechi, Wouter van Marlen Lichtenbelt, Denis Richard. Brown and beige adipose tissue phenotype and metabolic potential in mice and men. J Appl Physiologu. 1985;124(2):482–469.
- 35. Malsziewska K, Kretowski A. Brown adipose tissue and its role in insulin and glucose homeostasis. Int J Mol Science. 2021;22(4):1530.
- 36. Brandao BB, Poojari A, Rabiee A. Thetmogenic fat: development,physiological function and therapeutic potential. Int J Mol Science. 2021;22(11)5306.

