Introduction:Urge urinary incontinence (UUI) is a chronic debilitant condition that characterize some overactive bladder (OAB) patients in absence of any urinary tract infection (UTI). In our center we use intradetrusor injection of ‘Onabotulinum toxin A’ as second step after medical therapy failure in men who have underwent also to endoscopic surgery for BPH. The objective of this study is to verify the improvement in the patients' quality of life (QoL) and also to evaluate the effective dose over time.
Materials and Methods:We observed 40 male patients between January 2019 and January 2021, previously treated with oral drugs (anti-muscarinic and/or beta-3 adrenergic) and with surgical endoscopic approach (TURP, HoLEP, ThuLEP), 10 of these had pathologies - 4 of them neurological. These last patients were injected by 200U, all the others (36) by 100U - Botox®; Allergan, Irvine, CA, USA. Follow-up included monitoring of the following parameters at 3,6,12,24 months: urinary leaks (PAD test), Clean Intermittent Catheterization (CIC), OAB questionnaires, side effects. All patients underwent urodynamic examination before and 6 months after injection.
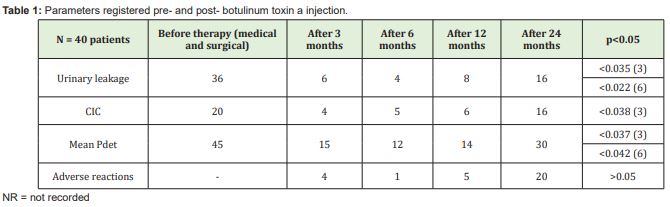
Results:The mean age was 60 years. 2 patients (5%) had early adverse effects after injection (1 vomiting, 1 pelvic pain), 4 (10%) needed CIC at 3 months, 1 of them also at 6 months (he was among the 4 neurological patients who underwent 200U dose). 38 (95%) answered positively to the questionnaire. Botox® treatment showed a reduction in urinary leakage at 3 and 6 months compared to medical therapy and a significant lowering of Pdet at 6 months (p<0.05). Both 100U and 200U doses proved to be effective up to one year after endoscopic treatment (p<0.05). The side effects at 3 months were 1 haematuria and 3UTI - 1 also recurred at 6 and 12 months
Keywords: Urge urinary incontinence, Over active bladder, Botox®, Intradetrusor injection, Benign prostatic hyperplasia
Abbreviations: 5-ARI: 5-Alpha Reductase Inhibitor; AUA: American Urological Association; BPH: Benign Prostatic Hyperplasia; CIC: Clean Intermittent Catheterization; EAU: European Association of Urology; ICS: International Continence Society; IIQ-7: Incontinence Impact Questionnaire; IPSS: International Prostate Symptom Score; IUSS: Indevus Urgency Severity Scale; KHQ: King’ Health Questionnaire; LUTS: Lower Urinary Tract Symptoms; OAB: Over Active Bladder (i-: idiopathic; n-: neurogenic; -q: questionnaires); OABSS: Overactive Bladder Symptoms Score; PPBC: Patient Perception of Bladder Condition questionnaire; PSA: Prostate-Specific Antigen; PVR: Post-Void Residual urine; Qmax: maximum peak flow; QoL: Quality of Life; SNM: Sacral Neuro Modulation; SUI: Stress Urinary Incontinence; UDI-6: Urogenital Distress Inventory; UTI: Urinary Tract Infection; UUI: Urge Urinary Incontinence
Urge Urinary Incontinence (UUI) is an annoying condition in which patients have to empty some urine volumes suddenly and with urgency.1
Over Active Bladder (OAB) is defined by International Continence Society (ICS) as urgency with or without UUI but usually with urinary frequency and nocturia in the absence of any Urinary Tract Infection (UTI).2 OAB can be divided into two groups: idiopathic (iOAB) or neurogenic (nOAB) if there is an underlying neurological disease, such Parkinson’s disease, ecc.3
There are many different data about the prevalence of OAB and the distribution between women and men, but most of the studies agree on the fact that it impacts public health especially in the elderly population, which is constantly increasing.4
Bladder capacity can be determined by ‘1-2-3 days’ diary, uroflowmetry with ultrasound control of Post-Void Residual (PVR) volume, cystometry,5 cystoscopy, but OAB is usually diagnosed after performing a complete urodynamic examination; some questionnaires can help to assess better this condition and can represent tools for treatment6: e.g. Overactive Bladder Symptoms Score (OABSS),7 Indevus Urgency Severity Scale (IUSS),8,9 International Prostate Symptom Score (IPSS)10, Urogenital Distress Inventory (UDI-6), Incontinence Impact Questionnaire (IIQ-7)11, King’ Health Questionnaire (KHQ)12, Patient Perception of Bladder Condition questionnaire (PPBC)13.
A conservative management is considered the first-line treatment, with the aim of reducing urinary frequency and increasing bladder volume. These therapies include lifestyle interventions such as fluid management, control of voiding time, urge suppression techniques, pelvic floor muscle physiotherapy to interrupt detrusor contractions. Second-line treatment includes oral pharmacotherapy: anti-muscarinic or β3-adrenoreceptor agonist.14 Several antimuscarinic drugs have been studied: oxybutynin, tolterodine, solifenacin, darifenacin, trospium, fesoterodine, propiverine, imipramine.15-20 Oxybutynin is a lipophilic tertiary amine that is activated by first-pass metabolism and has direct muscle relaxant effect, local anaesthetic and antimuscarinic properties with high affinity for muscarinic receptors in the bladder.21 It can be added on treatment patients refractory to one other antimuscarinic drug.22
Tolterodine’s active liver metabolite presents similar selectivity for bladder muscarinic receptors.23,24 Several trials have demonstrated a significant reduction in frequency and incontinent episodes, safety and efficacy were also compared to placebo and oxybutynin;25-30 mthis treatment can increase bladder capacity.31-32
Solifenacin demonstrated its efficacy on reducing urgency and PVR and on improving maximum peak flow (Qmax), although less potent than oxybutynin and tolterodine.33-36 Darifenacin is an effective tertiary amine, presenting moderate adverse events.37,38 Trospium chloride is a quaternary ammonium compound non-selective for muscarinic receptors, but some trials have demonstrated improvements in maximum cystometric capacity in comparison to placebo and similar oxybutynin effect in increasing bladder capacity and reducing maximum voiding detrusor pressure.39-42
Fesoterodine was considered the best anticholinergic by a 2021 multicriteria decision analysis model and better than the β3 adrenoceptor agonist mirabegron and solife- nacin/mirabegron drug combinations43. Propiverine has shown to increase bladder capacity and compliance, combining anticholinergic and calcium channel blocking actions.44-46 Imipramine and other tricyclic antidepressants can be used for their anticholinergic activities and capacity to decrease bladder contractility.47-49
The most common side effects of antimuscarinic drugs are dry mouth, constipation, blurred vision50. Mirabegron was the first β3-adrenoreceptor agonist approved for OAB treatment, showing similar efficacy to antimuscarinic drugs and fewer adverse effects.51-55 Association therapy with antimuscarinic plus β3-adrenoreceptor agonist can be considered after monotherapy for improving efficacy with low rates of side effects. Sacral neuromodulation (SNM), posterior tibial nerve stimulation (PTNS), augmentation cystoplasty and some other emerging therapies could be considered.57 Onabotulinum toxin A intradetrusor injection is an effective treatment in patients refractory to antimuscarinics.58
Botulinum toxin is a potent neurotoxin produced by Clostridium botulinum, Gram-negative anaerobic bacteria, that inhibits calcium-mediated release of acetylcholine vesicles at the presynaptic neuromuscular junction acting on peripheral cholinergic nerve endings.59 Seven serologic forms of botulinum toxin exist, but serotype A is the most commonly used for medical applications.60 Every toxin presents a heavy and light chain linked by a disulfide bond.61,62. The heavy chain determines connection specificity, while the light chain consists of the intracellular toxic portion.63-64 After internalitazion of the heavy and light chains in the neuronal cell, a disulfide reaction separates the chains and the light ones bind to the acetylcholine vesicles, acting as a zinc-dependent endopeptidase to cleave peptide bonds and prevent acetylcholine vesicle fusion with the plasma membrane, so inhibiting acetylcholine exocytosis and neurotransmission at the presynaptic junction that results in a flaccid paralysis of the muscle.65-67
Many articles have described and reviewed its use, indications, dose, administration, success rates and limitations. In our center we use intradetrusor injection of ‘Onabotulinum toxin A’ as second step after medical therapy failure in men who have underwent also to endoscopic surgery for Benign Prostatic Hyperplasia (BPH).
The aim of this study was to verify the improvement in the patients' quality of life (QoL) and also to evaluate the effective dose over time, in order to plan future treatments carefully.
Patient selection
This study included 40 consecutive male patients between January 2019 and January 2021, all with urodynamic diagnosis of OAB and previously treated with oral drugs (anti-muscarinic and/or β3 adrenergic).
10 of these patients (25%) presented some other phatologies, 4 of them have neurological diseases resulting as nOAB (10%), the other ones were iOAB patients (90%).
These 40 men had also irritative and obstructive Lower Urinary Tract Symptoms (LUTS) non-responsive to alpha-litic drugs (alfuzosin, silodosin, tamsulosin) and to 5-ARI (dutasteride, finasteride), so they underwent to endoscopic disobstructive interventions before toxin injection, sometimes also in other centers (28 ThuLEPs, 8 TURPs, 4 HoLEPs).
Parameters Measured
The following aspects were considered pre- and post-procedure at 3,6,12,24 months, in order to assess the efficiency of the technique: if there were any urinary leaks, the measure of the eventual leakage by considering the PAD test, if Clean Intermittent Catheterization (CIC) was necessary, Quality of Life (QoL) was assessed by OAB questionnaires, all complications were registered.
Description of the Technique
Equipment
- Onabotulinum toxin A (100U)
- Rigid cystoscope (telescope 70º/0º, bridge, and sheath)
- Needle 22-27 Gauge and 4mm (70cm)
- Two Syringes 10ml
- Two Lidocaine 2% 10ml
- Sodium Bicarbonate (8.4%) Injection (10ml)
- Lidocaine gel
- Foley 16Ch
- Two Saline solutions 10ml
- One Saline solution 50ml
Doses
The most common doses utilised were 100 UI for iOAB and 200UI for nOAB.
Surgical technique
The patient is placed in the lithotomy position. A gentle OTIS urethrotomy is performed if necessary. Rigid cystoscope is positioned and a 22G needle is inserted into the bladder wall and withdrawn halfway prior to injection.68 After local anaesthesia, a fine sheath (27G) is introduced through the working channel of the cystoscope and the fine needle is passed through the sheath.69,70 20 injections are performed throughout the bladder, trigone sparing in iOAB patients, trigone inclusive (5 in the trigone, 15 outside the trigone) for nOAB ones who need CIC.71
Statistical analysis
Data were collected using Microsoft Excel (version 12.2.4) and analysed with SPSS (version 22.0). Statistical differences in means were determined with t-tests; the significance level was set at p<0.05.
The mean age was 60.0 years. 2 patients (5%) had early adverse effects after injection (1 vomiting, 1 pelvic pain), 4 (10%) needed CIC at 3 months, 1 of them also at 6 months (they were among the 8 neurological patients who underwent 200U dose). 38 (95%) answered positively to the questionnaire.
Botox® treatment showed a reduction in urinary leakage at 3 and 6 months after medical therapy failure and surgical procedure, and a significant lowering of Pdet at 6 months (p<0.05). Both 100 U and 200 U doses proved to be effective up to one year after toxin injection (p<0.05).
The side effects at 3 months were 1 haematuria and 3 UTI - 1 also recurred at 6 and 12 months - there was no statistically significant difference with oral drugs.
Table 1 shows results.
We believe that every correct approach to a disease goes step by step and certainly starting from the least invasive, from this point of view we agree on the fact that primarily OAB is correctly diagnosed by urodynamic examination and then treated primarily with lifestyle and physiotherapy, starting a medical therapy in conjunction with or immediately after the ineffectiveness or partial effectiveness of the conservative behavioral treatment; pelvic floor physiotherapy can be considered also.4;72-87
If the medical therapy has not had the desired effect, we perform the intratrusor treatment with botulinum toxin, which has been shown to have good efficacy.88-93
We think it is safer to perform this procedure in the face of a negative urine culture, although Bickhaus they stated that performing a first injection of onabotulinum toxin A within 30 days of urinary tract infection does not increase the odds of post-procedure urinary tract infection.94
Huang showed that intradetrusor injection of onabotulinum toxin A at the time of HoLEP is safe and is associated with improved urinary incontinence scores and AUA Symptom Score.95 We performed toxin injection after the endoscopic disobstructive prostatic operation, because sometimes we observed the patient for persistent LUTS after the operations or to wait till the complete resolution of inflammatory field.
Chugtai assessed the efficacy of onabotulinumtoxinA (BOTOX, Allergan Inc., Irvine, CA, USA) in patients with refractory overactive bladder (OAB) after treatment for benign prostatic hyperplasia (BPH), although improvements in QoL were not statistically significant.96
Moussa demonstrated that intraprostatic injection of botulinum toxin A as modality treatment of LUTS/BPH significantly improved IPSS, Qmax, PVR, and decreased prostate volume.97
Similar results were reached also by Totaro and Ding.98,99
We have decided to administer 100U, while 200U have been injected in neurological patients. Abdelwahab highlighted that 100U injections seemed to have comparable results with 200U.100 Arnouk randomized 2 groups of men with symptomatic BPH who failed medical treatment to receive 100U or 200U of BoNT-A into the prostate; the International Prostatic Symptom Score (IPSS), maximum flow rate (Q(max)), post-void residual volume (PVR), PSA levels and prostate volume before injection and after 3 and 6 months were evaluated. It was demonstrated that both doses produced significant improvements in IPSS, Q(max) and PVR after 3 and 6 months and both doses promoted comparable effects. Prostate volume was affected by 200U BoNT-A injection only after 6 months of treatment. PSA levels were significantly affected in the 100U group only after 6 months of treatment. In the 200U group, PSA levels were significantly decreased after 3 and 6 months. The complication rate was similar in both groups.101
The effectiveness of using this therapy has also been studied in cases following other surgical procedures, such as midurethral sling in women.102
The use of botulinum toxin A has been shown to improve urodynamic outcomes and the quality of life of patients, however its efficacy lasts up to a certain period and this must lead to the creation of a sort of programme/calendar with reminders of the treatment, stratify them according to the patients and anticipating the more complicated ones such as neurological ones.
Even if they were patients with prostatic hyperplasia, our population was quite heterogeneous, and this can be a pro to the extent that we have seen that the therapy is effective on neurological patients or not, a limitation can be given by the small number of the sample and by not having considered other parameters such as race or different surgical endoscopic operations or if they had undergone physiotherapy or other types of minimally invasive therapy before.
In general Botox® seems a valid therapeutic option for OAB patients, in particular after medical therapy failure. 100U appears as an effective dose, but we prefer to use 200U in patients with neurological diseases. There were no clinical relevant differences between the doses, however a loss of effect is observed after 12 months the last intradetrusorial injection; this means that the treatment must be repeated after about one year, and possibly anticipated in neurological patients. Intradetrusor injection of ‘onabotulinum toxin A’ can be applied also in men affected by BPH who have previously undergone endoscopic prostatic surgery. Other clinical detections and considerations should be assessed in the next future.
All 40 patients signed an informed consent declaring to have understood the purposes, benefits, and risks of the proposed treatments.
None.
None.
None.
- 1. Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327-336.
- 2. Abrams P, Artibani W, Yanping Zhang, et al. International Continence Society: reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn. 2009;28:287.
- 3. Ji, Erpeng Liu, Jin Guo Wen. Mechanism and Priority of Botulinum Neurotoxin A versus Sacral Neuromodulation for Refractory Overactive Bladder: A Review. Urol Int. 2021;105:929-934.
- 4. Truzzi JC, Mendes CG, Bezerra CA, et al. Overactive bladder-18 years-Part I. Int Braz J Urol. 2016;42(2):188-198.
- 5. Ertberg P, Moller LA, Lose G. A comparison of three methods to evaluate maximum bladder capacity: cystometry, uroflowmetry and a 24-h voiding diary in women with urinary incontinence. Acta Obstet Gynecol Scand. 2003;82:374-377.
- 6. Sheng Mou Hsiao, Ho Hsiung Lin. Medical treatment of female overactive bladder syndrome and treatment-related effects. Review Article. Journal of the Formosan Medical Association. 2018;117:871-878.
- 7. Homma Y, Yoshida M, Seki N, et al. Symptom assessment tool for overactive bladder syndrome-overactive bladder symptom score. Urology. 2006;68:318-323.
- 8. Nixon A, Colman S, Sabounjian L, et al. A validated patient reported measure of urinary urgency severity in overactive bladder for use in clinical trials. J Urol. 2005;174:604-607.
- 9. Chung SD, Liao CH, Chen YC, et al. Urgency severity scale could predict urodynamic detrusor overactivity in patients with overactive bladder syndrome. Neurol Urodyn. 2011;30:1300-1304.
- 10. Barry MJ, Fowler Jr FJ, O’Leary MP, et al. The American Urological association symptom index for benign prostatic hyperplasia. The measurement committee of the American Urological Association. J Urol. 1992;148:1549-1557
- 11. Uebersax JS, Wyman JF, Shumaker SA, et al. Short forms to assess life quality and symptom distress for urinary incontinence in women: the incontinence impact questionnaire and the Urogenital distress inventory. Continence program for women research group. Neurourol Urodyn. 1995;14:131-139.
- 12. Kelleher CJ, Cardozo LD, Khullar V, et al. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol. 1997;104:1374-1379.
- 13. Coyne KS, Matza LS, Kopp Z, et al. The validation of the patient perception of bladder condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol. 2006;49:1079-1086.
- 14. Toya S Pratt, Anne M Suskind. Management of Overactive Bladder in Older Women. Curr Urol Rep. 2019;19(11):92.
- 15. Abrams P, Andersson KE, Buccafusco JJ, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148:565-578
- 16. Hegde SS. Muscarinic receptors in the bladder: from basic research to therapeutics. Br J Pharmacol. 2006;147:S80-S87.
- 17. Chancellor MB, Levanovich P, Rajaganapathy BR, et al. Optimum management of overactive bladder: medication vs BotoxTM vs InterStimTM vs UrgentTM PC. Urol Pract. 2014;1:7-12.
- 18. Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188:2455-2463.
- 19. Hood B, Andersson KE. Common theme for drugs effective in overactive bladder treatment: inhibition of afferent signaling from the bladder. Int J Urol. 2013;20:21-27.
- 20. Roxburgh C, Cook J, Dublin N. Anticholinergic drugs versus other medications for overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2007;3:CD003190.
- 21. Nilvebrant L, Andersson KE, Mattiasson A. Characterization of the muscarinic cholinoreceptors in the human detrusor. J Urol. 1985;134:418-23.
- 22. Wang CC, Jiang YH, Kuo HC. Efficacy and adherence of flexibly addind on a second antimuscarinic agent for patients with refractory overactive bladder. Low Urin Tract Symptoms. 2017;9:27-32.
- 23. Ruscin JM, Morgenstern NE. Tolterodine use for symptoms of overactive bladder. Ann Pharmacoter. 1999;33:1073-1082
- 24. Nilvebrant L, Hallen B, Larsson G. Tolterodine – a new bladder selective muscarinic receptor antagonist: preclinical pharmacological and clinical data. Life Sci. 1997;60:1129-1136.
- 25. Hills CJ, Winter SA, Balfour JA. Tolterodine. Drugs. 1998;55:813-820.
- 26. Jonas U, Hofner K, Madesbacher H, et al. Efficacy and safety of two doses of tolterodine versus placebo in patients with detrusor overactivity and symptoms of frequency, urge incontinence, and urgency: urodynamic evaluation. World J Urol. 1997;15:144-151.
- 27. Millard R, Tuttle J, Moore K, et al. Clinical efficacy and safety of tolterodine compared to placebo in detrusor overactivity. J Urol. 1999;161:1551-1555.
- 28. Rentzog L, Stanton SL, Cardozo LD, et al. Efficacy and safety of tolterodine in patients with detrusor instability: a dose ranging study. Br J Urol. 1998;81:42-48.
- 29. Abrams P, Freeman R, Anderstrom C, et al. Tolterodine, a new antimuscarinic agent: as effective but better tolerated than oxybutynin in patients with an overactive bladder. Br J Urol. 1998;81:801-810.
- 30. Appel RA. Clinical efficacy and safety of tolterodine in the treatment of overactive bladder: a pooled analysis. Urology. 1997;50:90-96.
- 31. Wu WY, Hsiao SM, Chang TC, et al. Changes in urodynamic parameters after tolterodine treatment for female overactive bladder syndrome with or without voiding dysfunction. J Obstet Gynaecol Res. 2011;37:436-441
- 32. Wang CL, Wu CH, Liu CM, et al. Clinical and urodynamic effects of tolterodine in women with an overactive bladder. Taiwan J Obstet Gynecol. 2013;52:381-384.
- 33. Hsiao SM, Lin HH, Kuo HC. Factors associated with a better therapeutic effect of solifenacin in patients with overactive bladder syndrome. Neurourol Urodyn. 2014;33:331-334.
- 34. Cardozo L, Lisec M, Millard R, et al. Randomised, double blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol. 2004;172:1919-1924.
- 35. Chapple CR, Arano P, Bosch JL, et al. Solifenacin appears effective and well tolerated in patients with symptomatic idiopathic detrusor overactivity in a placebo and tolterodine controlled phase II dose-finding study. BJU Int. 2004;93:71-77.
- 36. Chapple CR, Martinez Garcia R, Selvaggi L, for the STAR study group, et al. A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: results of the STAR trial. Eur Urol. 2005;48:464-470.
- 37. Haab F, Stewart L, Dwyer P. Darifenacin, an M3 selective receptor antagonist, is an effective and well-tolerated once-daily treatment for overactive bladder. Eur Urol. 2004;45:420-429.
- 38. Chapple CR. Darifenacin is well tolerated and provides significant improvement in the symptoms of overactive bladder: a pooled analysis of phase III studies. J Urol. 2004;171:130.
- 39. Schladitz Keil G, Spahn H, Mutschler E. Determination of bioavailability of the quaternary ammonium compound trospium chloride in man from urinary excretion data. Arzneimittel Forsh/Drug Res. 1986;36:984-987.
- 40. Fusgen I, Hauri D. Trospium chloride: an effective option for medical treatment of bladder overactivity. Int J Clin Pharmacol Ther. 2000;38:223-234.
- 41. Cardozo LD, Chapple CR, Toozs Hobson P, et al. Efficacy of trospium chloride in patients with detrusor instability: a placebo-controlled, randomized, double-blind, multicentre clinical trial. BJU Int. 2000;85659-85664.
- 42. Madersbacher H, Stoher M, Richter R, et al. Trospium chloride versus oxybutyinin: a randomized, double-blind, multicentre trial in the treatment of detrusor hyperreflexia. Br J Urol. 1995;75:452-456.
- 43. Milsom I, Wagg A, Oelke M, Chapple C. Which drugs are best for overactive bladder? From patients’ expectations to physicians’ decisions. Int J Clin Pract. 2021;75:e13870.
- 44. Haruno A, Yamasaki Y, Miyoshi K, et al. Effects of propiverine hydrochloride and its metabolites on isolated guinea pig urinary bladder. Folia Pharmacol Japan. 1989;94:145-150.
- 45. Mazur D, Wehnert J, Dorschner W, et al. Clinical and urodynamic effects of propiverine in patients suffering from urgency and urge incontinence. Scand J Urol Nephrol. 1995;29:289-294.
- 46. Stoher M, Madersbacher H, Richter R, et al. Efficacy and safety of propiverine in SCI-patients suffering from detrusor hyperreflexia: a double-blind, placebo-controlled clinical trial. Spinal Cord. 1999;37:196-200.
- 47. Lin HH, Sheu BC, Lo MC, et al. Comparison of treatment outcomes for imipramine for female genuine stress incontinence. Br J Obstet Gynaecol. 1999;106:1089-1092.
- 48. Redaelli M, Ricatti MJ, Simonetto M, et al. Serotonin and noradrenaline reuptake inhibitors improve micturition control in mice. PLoS One. 2015;10:e0121883.
- 49. Wein AJ. Pharmacologic options for the overactive bladder. Urology. 1998;51(2A Suppl):43-47.
- 50. Srikrishna S, Robinson D, Cardozo L, et al. Management of overactive bladder syndrome. Postgrade Med J. 2007;83:481-486.
- 51. Hsiao SM, Chang TC, Chen CH, et al. Comparison of the clinical outcomes and urodynamic effects of mirabegron versus tolterodine treatment for female overactive bladder syndrome: a subgroup analysis of a controlled, randomised, prospective study. Low Urin Tract Symptoms. 2018;10(3):215-220.
- 52. Kuo HC, Lee KS, Na Y, et al. Results of a randomized, double-blind, parallel-group, placebo- and active-controlled, multicenter study of mirabegron, a β3-adrenoreceptor agonist, in patients with overactive bladder in Asia. Neurourol Urodyn. 2015;34:685-692.
- 53. Batista JE, Kolbl H, Herschorn S, et al. The efficacy and safety of mirabegron compared with solifenacin in overactive bladder patients dissatisfied with previous antimuscarinic treatment due to lack of efficacy: results of a noninferiority, randomized, phase IIIb trial. Ther Adv Urol. 2015;7:167-179.
- 54. Aballea S, Maman K, Thokagevistk K, et al. Cost effectiveness of mirabegron compared with tolterodine extended release for the treatment of adults with overactive bladder in the United Kingdom. Clin Drug Invest. 2015;35:83-93.
- 55. Nazir J, Maman K, Neine ME, et al. Cost-effectiveness of mirabegron compared with antimuscarinic agents for the treatment of adults with overactive bladder in the United Kingdom. Value Health. 2015;18:783-790.
- 56. Bunniran S, Davis C, Kristy R, et al. A prospective study of elderly initiating mirabegron versus antimuscarinics: patient reported outcomes from the Overacative Bladder Satisfaction Scales and other instruments. Neurourol Urodyn. 2018;37:177-185.
- 57. Fontaine C, Papworth E, Pascoe J, et al. Update on the management of overactive bladder. Ther Adv Urol. 2021;13:1-9.
- 58. Hsiao SM, Lin HH. Medical treatment of female overactive bladder syndrome and treatment-related effects. Review Article. Journal of the Formosan Medical Association. 2018;117:871-878.
- 59. Ermengem EV. Ueber einen neuen anaeroben Bacillus und seine Beziehungen zun Botulismus. Zeitschrift fur Hygiene und Infektionsk-rankheiten. 1897;26:1-56.
- 60. Mahajan ST, Brubaker L. Botulinum toxin: from life-threatening disease to novel medical therapy. Am J Obstet Gynecol. 2007;196:7-15.
- 61. Simpson L, Dasgupta B. Botulinum neurotoxin in type E: studies on the mechanism of action and on structure activity relationship. Pharmacol Exp Ther. 1983;224:135-140.
- 62. Simpson L. Kinetic studies on the interaction between botulinu toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther. 1980;212:16-21.
- 63. Simpson L. Peripheral actions of the botulinum toxins. In: Simpson L, ed. Botulinum neurotoxin and tetanus toxin. New York: Academic Press; 1989.
- 64. Zhou L, Paiva Ad, Liu D, et al. Expression and purification of the light chain of botulinum neurotoxin A: a single mutation abolishes its cleavage of SNAP 25 and neurotoxicity after reconstitution with the heavy chain. Biochem. 1995;34:15175-15181.
- 65. Dolly J, Black J, Williams R, et al. Acceptors for botulinum neurotoxin reside on motor nerve terminals and mediate its internalisation. Nature. 1984;307:457-460.
- 66. Stecher B, Weller U, Habermann E. The light chain but not the heavy chain of botulinum type A toxin inhibits exocytosis from permeabilised adrenal chromaffin cells. FEBS Lett. 1989;255:318-325.
- 67. Binder W, Brin M, Blitzer A, et al. Botulinum toxin type A (BOTOX) for treatment of migraine. Dis Mon. 2002;48:323-335.
- 68. Mehnert U, Boy S, Schimd M, et al. A morphological evaluation of botulinum neurotoxin A injections into the detrusor muscle using magnetic resonance imaging. World J Urol. 2009;27:397-403.
- 69. Harper M, Popat RB, Dasgupta R, et al. A minimally invasive technique for outpatient local anaesthetic administration of intradetrusor botulinum toxin in intractable detrusor overactivity. BJU Int. 2003; 92:325-326.
- 70. Seth JH, Dowson C, Khan MS, et al. Botulinum toxin-A for the treatment of overactive bladder: UK contributions. Journal of Clinical Urology. 2013;6(2):77-83.
- 71. Manecksha RP, Cullen IM, Ahmad S, et al. Prospective randomised controlled trial comparing trigone-sparing versus trigone-including intradetrusor injection of abobotulinumtoxinA for refractory idiopathic detrusor overactivity. Eur Urol. 2012;61:928-935.
- 72. Creighton SM, Stanton SL. Caffeine: does it affect your bladder? Br J Urol. 1990;66:613-614.
- 73. Arya LA, Myers DL, Jackson ND. Dietary caffeine intake and the risk for detrusor instability: a case-control study. Obstet Gynecol. 2000;96:85-89.
- 74. Holroyd-Leduc JM, Straus SE. Management of urinary incontinence in women: scientific review. JAMA. 2004;291:986-995.
- 75. Tomlinson BU, Dougherty MC, Pendergast JF, et al. Dietary caffeine, fluid intake and urinary incontinence in older rural women. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:22-28.
- 76. Bryant CM, Dowell CJ, Fairbrother G. Caffeine reduction education to improve urinary symptoms. Br J Nurs. 2002;11:560-565.
- 77. Wells MJ, Jamieson K, Markham TC, et al. The effect of caffeinated versus decaffeinated drinks on overactive bladder: a double-blind, randomized, crossover study. J Wound Ostomy Continence Nurs. 2014;41:371-378.
- 78. Swithinbank L, Hashim H, Abrams P. The effect of fluid intake on urinary symptoms in women. J Urol. 2005;174:187-189.
- 79. Hashim H, Abrams P. How should patients with an overactive bladder manipulate their fluid intake? BJU Int. 2008;102:62-66.
- 80. Ouslander JG, Schnelle JF. Incontinence in the nursing home. Ann Intern Med. 1995;122:438-449.
- 81. Fantl JA, Wyman JF, McClish DK, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA. 1991;265:609-613.
- 82. Burgio KL, Kraus SR, Borello France D, et al. Urinary Incontinence Treatment Network. The effects of drug and behavior therapy on urgency and voiding frequency. Int Urogynecol J. 2010;21:711-719.
- 83. Rai BP, Cody JD, Alhasso A, et al. Anticholinergic drugs versus non-drug active therapies for non-neurogenic overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2012;12:CD003193.
- 84. Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles. Am J Obstet Gynecol. 1948;56:238-248.
- 85. Kafri R, Deutscher D, Shames J, et al. Randomized trial of a comparison of rehabilitation or drug therapy for urgency urinary incontinence: 1-year follow-up. Int Urogynecol J. 2013;24:1181-1189.
- 86. Borello France D, Burgio KL, Goode PS, et al. Urinary Incontinence Treatment Network. Adherence to behavioral interventions for urge incontinence when combined with drug therapy: adherence rates, barriers, and predictors. Phys Ther. 2010;90:1493-505.
- 87. Lamb SE, Pepper J, Lall R, et al. Group treatments for sensitive health care problems: a randomised controlled trial of group versus individual physiotherapy sessions for female urinary incontinence. BMC Womens Health. 2009;9:26.
- 88. Rovner E, Dmochowski R, Chapple C, et al. OnabotulinumtoxinA improves urodynamic outcomes in patients with neurogenic detrusor overactivity. Neurology and Urodynamics. 2013;32:1109-1115.
- 89. Wang CC, Lee CL, Kuo HC. Efficacy and safety of intravescical Onabotulinum toxinA injection in patients with detrusor hyperactivity and impaired contractility. Toxins. 2016;8:82.
- 90. Joussain C, Phe V, Even A, et al. Intradetrusor injection of botulinum toxin A and sacral neuromodulation for neurogenic detrusor overactivity. European Journal of Physical and Rehabilitation Medicine. 2017;53(6):991-997.
- 91. Jiang YH, Yu WR, Kuo HC. Therapeutic effect of botulinum toxin A on sensory bladder disorders – from bench to bedside. Toxins. 2020;12:166.
- 92. Wang CC, Chou ECL, Chuang YC, et al. Effectiveness and safety of intradetrusor OnabotulinumtoxinA injection for neurogenic detrusor overactivity and overactive bladder patients in Taiwan – A phase IV prospective, interventional, multiple-center study (Restore Study). Toxins. 2021,13:911.
- 93. Chancellor MB, Migliaccio Walle K, Bramley TJ, et al. Long-term patterns of use and treatment failure with anticholinergic agents for overactive bladder. Clinical Therapeutics. 2013;35:1744-1751.
- 94. Bickhaus JA, Bradley MS, Amundsen CL, et al. Does a recent Urinary Tract Infection increase the risk of post-procedure Urinary Tract Infection after Onabotulinum Toxin A? Female Pelvic Med Reconstr Surg. 2021;27(2):121-125.
- 95. Huang MM, Dean NS, Assmus MA, et al. Intradetrusor OnabotulinumtoxinA Injections at the Time of Holmium Laser Enucleation of the Prostate for Men with Severe Storage Symptoms. J Endourol. 2023;37(7):801-806.
- 96. Chughtai B, Dunphy C, Lee R, et al. Randomized, double-blind, placebo controlled pilot study of intradetrusor injections of onabotulinumtoxinA for the treatment of refractory overactive bladder persisting following surgical management of benign prostatic hyperplasia. Can J Urol. 2014;21(2):7217-7221.
- 97. Moussa AS, Ragheb AM, Abdelbary AM, etal. Outcome of Botulinum Toxin-A intraprostatic injection for benign prostatic hyperplasia induced lower urinary tract symptoms: A prospective multicenter study. Prostate. 2019;79(11):1221-1225.
- 98. Totaro A, Pinto F, Pugliese D, et al. Intraprostatic botulinum toxin type "A" injection in patients with benign prostatic hyperplasia and unsatisfactory response to medical therapy: A randomized, double-blind, controlled trial using urodynamic evaluation. Neurourol Urodyn. 2018;37(3):1031-1038.
- 99. Ding XD, Chen HX, Xiao HQ, et al. Treatment of Benign Prostatic Hyperplasia by Ultrasound-Guided Botulinum Toxin Type A Injection. Cell Biochem Biophys. 2015;73(2):357-359.
- 100. Abdelwahab O, Sherif H, Soliman T, et al. Efficacy of botulinum toxin type A 100 units versus 200 units for treatment of refractory idiopathic overactive bladder. Int Braz J Urol. 2015;41:1132-1140.
- 101. Arnouk R, Suzuki Bellucci CH, Benatuil Stull R, et al. Botulinum neurotoxin type A for the treatment of benign prostatic hyperplasia: randomized study comparing two doses. Scientific World Journal. 2012;2012:463574.
- 102. Miotla P, Futyma K, Cartwright R, et al. Effectiveness of botulinum toxin injection in the treatment of de novo OAB symptoms following midurethral sling surgery. Int Urogynecol J. 2016;27:393-408.

