Background: Cardiovascular events are the leading cause of morbidity and mortality in chronic hemodialysis patients. Impaired right ventricular function is often associated with poor survival in hemodialysis patients.
Objective: To determine the effect of vascular access flow (AVFs) of right ventricular function.
Methods: This cross-sectional study was done in Department of Nephrology, DMCH during the period January 2020 to July 2021. End stage renal disease patient underwent hemodialysis for ≥3 months were recruited in the study. Patients with chronic respiratory disease, severe valvar heart disease, AVFs flow<200ml/min were excluded. Right ventricular function was assessed by Tricuspid Annular Plane Systolic Excursion (TAPSE) through echocardiography and AVFs blood flow by color duplex study. History for diabetes mellitus, glomerulonephritis and hypertension were retrieved from medical records in addition to variable age and gender. Results of the biochemical variable also retrieved. TAPSE ≤16mm was taken as cut off to characterize subjects as Group I, impaired right ventricular function. Those with >16mm termed as Group II, normal right ventricular function. Data were expressed as mean±SD and number (present) as appropriate. Unpaired student t test, invariable and multivariable analysis were performed as applicable using statistical package for social science (SPSS). p<0.05% was taken as level of significant.
Results: of the total 80 subject 26 constituted Group I and 54 in Group II. Distribution of male and female did not show any statistical association. Distribution of gender in two groups did not show any statistical association. Distribution of the subjects in age cluster was similar in the two groups. History of DM, GN, and HTN distribution in the two groups was also similar. Duration of hemodialysis (mean±SD) in Group I was 28.04 months and 26.39 months in Group II; two group did not show statistical difference (p=0.529). Systolic blood pressure was 163 mmHg and 160mmHg in Group I and Group II respectively and diastolic blood pressure (mean±SD, mmHg) was 92mmHg and 92mmHg in Group I and Group II respectively; did not statistical difference (p=0.283 and 0.960 respectively). AVFs blood flow 1603±382ml/min (mean±SD,ml/min) in group I was significantly higher compared to the counterpart group II 791±189ml/min. The trend was supported by the significant (p=0.001) inverse relationship between TAPSE and AVFs blood flow. In Group I radio cephalic and brachiocephalic AV fistula distribution was 12 vs 24 (46.2 vs 53.8%) which in the Group II was 51 vs 3(94.4 vs 5.6%).
Conclusion: Data concluded that brachiocephalic AVFs lead to significantly higher blood flow than the radio cephalic fistula resulting impaired right ventricular function, which reconfirmed the usefulness of distal AVF as a vascular access in patient for hemodialysis.
Keywords: Hemodialysis, Right ventricular function, Vascular access, Radio cephalic fistula, Brachiocephalic fistula
End Stage Renal Disease (ESRD) patients are more prone to cardiovascular diseases. According to United States Renal Data System (USRDS) 2017, cardiovascular complications are relatively more common in hemodialysis (69.8%) compare to peritoneal dialysis (56.6%) and renal transplant patients (41.6%). Arteriovenous fistula used in patient of end stage renal disease for hemodialysis since 1960s.1 There is a trend toward use of the arteriovenous fistula as a initial choice rather than central venous catheters.2 After formation of arteriovenous fistula there is increase in after load due to shunt which leads to right ventricular hypertrophy. Right ventricular hypertrophy reduce ventricular compliance and lead to left ventricular filling defect via interventricular Interaction.3 Risk factors for cardiovascular diseases in Chronic Kidney Disease (CKD) patient attributed to traditional and nontraditional variety. Nontraditional risk factors such as albuminuria, homocysteine, anemia, abnormal calcium/phosphate metabolism, extracellular fluid overload found to impart in development of cardiovascular diseases.4 It mainly manifests in the form of ventricular hypertrophy, myocardial fibrosis, valvulopathies, arrhythmias and sudden death are more common in Chronic Kidney Disease Patient.5 Renal transplantation conceptually is the treatment of choice for Chronic Kidney Disease (CKD) patients with End Stage Renal Disease (ESRD).6 It was observed that 50% CKD patient already develop cardiac disease by the time they started dialysis.7 During interim period or in absence of transplantation choice for patient include hemodialysis and peritoneal dialysis. According to National Institute of Diabetes and Digestive and Kidney disease (NIH) during 2013, 63.7% of ESRD patient received hemodialysis, 6.8% received peritoneal dialysis and 29.2% patient received renal transplantation.
The first patient treated with PD was reported by Ganter in 1923. The patient was a female with ureteral obstruction that was due to uterine carcinoma. In 1938, Rhoads used intermittent dialysis (IPD) to treat two nephrotic patients using dwell times of 50 minutes and total dialysate volumes of 6 – 11 L. Boen in 1964 developed automated dialysate delivery system for peritoneal dialysis. Hospital-based intermittent chronic peritoneal dialysis (IPD) is the oldest PD modality, applied for as long as 40 h per week, using high volumes of PD fluids. With the advent and improvements of continuous ambulatory (CAPD) and automated peritoneal dialysis (APD), the method has been criticized for its long duration and low adequacy regarding solute clearances and has almost been abandoned in western countries (History of PD, 2014). Advantages of peritoneal dialysis include patient autonomy, maintenance of residual renal function, patient satisfaction, less cost, less delayed graft function post transplantation, survival years 1 to 2 years. Disadvantages include high technique failure rate, weight gain, and patient and caregiver burnout.8
The first human hemodialysis was performed in a uremic patient by Haas in 1924 at the University of Giessen in Germany Haas.9,10 while taking care of casualties after the German invasion of the Netherlands, his interest in acute renal failure further increased and in 1943 he introduced the rotating drum hemodialysis system using cellophane membranes and an immersion bath and the first recovery of an acute renal failure patient treated with hemodialysis was reported Kolff.10 This was the beginning of what was to become an important clinical reality: artificial renal substitution therapy. Advantages of hemodialysis include less patient responsibility, community. Disadvantages includes infection, access complication, high mortality just before and 12 hours after treatment possibly due to electrolyte issues.8 A new phase in clinical hemodialysis started with the introduction of the Quinton and Scribner arteriovenous shunt.11 They used silastic tubes fitted with Teflon tips into the radial artery and cephalic vein in the wrist or the posterior tibial artery and saphenous vein at the angle as an arteriovenous shunt. The two tubes ended in expanded couplings to facilitate connection. This shunt provided for the first time continuous circulation of the blood when the patient was not attached to the machine, effectively eliminating clotting and provided ready access for repeated long-term hemodialysis, opening the door to chronic renal replacement therapy.
The next significant advance in vascular access took place later in the 1960s when Cimino and Brescia1 first described their native arteriovenous fistula for chronic vascular access.1 these fistulas are generally created by an end-to-side vein-to-artery anastomosis allowing access for hemodialysis. A mature native arteriovenous fistula is by far found to be the safest and longest lasting vascular access for hemodialysis mature wrist arteriovenous radio cephalic fistula usually shown to have blood flow around 500-800ml/min vs excess of 1000ml/min in fistula at antecubital fossa brachiocephalic fistula.12 Uses of arteriovenous fistulas (AVFs) as a vascular access increases nearly double from 32% in 2003 to 60% in 2011 because AVFs have less complication in compared to other vascular access.2 Despite its high success rate for long‑term hemodialysis access, AVFs found to be complicated by flow derangements.13
Right ventricular dysfunction in chronic hemodialysis patient may be due to chronic volume overload, uremia and this dysfunction increases further by AVFs through increasing preload. In a study, it has been shown that right ventricular dysfunction (assess by right ventricular chamber dilatation and tricuspid annular plane systolic excursion–TAPSE) more in patients hemodialysis through AVFs compared through Central venous catheter.14 a study by Kjaergaard, 15 right ventricular function is important predictor of survival in patient with new onset or worsening heart failure and 50% increase in TAPSE value leads to one fourth reduction of mortality. A study by Zhao16 showed that right ventricular dysfunction may be caused by volume overload due to AVFs. They showed that nearly 50percent of chronic hemodialysis patient have right ventricular dysfunction. For evaluation of right ventricular systolic function there are several echocardiographic parameter. TAPSE (Tricuspid annular plane systolic excursion) is one of the parameter. It measures distance of the tricuspid valve movement from the base of the heart towards the apex. The importance of evaluation of TAPSE is that it is simple and easily done.17 chronic heart failure can develop due to volume overload between dialysis sessions, sympathetic discharge, ventricular hypertrophy, anemia, arterial stiffness, and vascular access flow. Each one of these mechanisms may partly contribute to an increase in either preload or after load of both ventricles.18 Paneni19 showed that right ventricular dysfunction more common in hemodialysis group compared to peritoneal dialysis group (71.3% vs 34.6%), especially in brachiocephalic AVFs. Pulmonary hypertension in patients on hemodialysis has been extensively studied. But data on the development of right ventricular dysfunction in relation to AVFs blood flow ESRD patients is scarce. The purpose of the study is to find out relationship between vascular access flow with impaired right ventricular function assess by TAPSE.
This cross-sectional study was conducted in the Department of Nephrology, Dhaka Medical College Hospital, Dhaka, Bangladesh from January 2020 to July 2021 among eighty patients (80) who were diagnosed with ESRD and who were receiving 2 or more hemodialysis session per week through AV fistula for at least 3 months. All participants were explained about the natural history, pathophysiology, relevant investigations, current treatment options and outcome of ESRD prior to enrollment.
Inclusion criteria
Patients with 2 or more hemodialysis session per week through AV fistula for at least 3 months
Adult male and female patients with age between 18 and 65 years
Exclusion criteria
Arteriovenous fistula blood flow (<200ml/min)
Patient’s with:
- Severe chronic obstructive pulmonary disease,
- History of pulmonary embolism,
- Primary pulmonary hypertension,
- Recent myocardial infarction (<1month),
- Unstable angina pectoris,
- History of heart valve surgery,
- Severe mitral, aortic or pulmonary regurgitation, and/or stenosis
Who are not willing to participate in the study
CKD patients taking maintenance hemodialysis in the hemodialysis unit or admitted into the inpatient department of nephrology department of DMCH were invited to participate. Selection of patient was done as per inclusion and exclusion criteria. All patients underwent a transthoracic echocardiography (TTE) which was performed by cardiologist. Color Duplex examination was performed by a radiologist. Then patient was divided into two group based on TAPSE (Tricuspid Annular Pan Systolic Excursion) Group I impaired right ventricular function (TAPSE<16mm) and Group II Normal right ventricular function (TAPSE>16mm). All data were analyzed by SPSS 22.0(SPSS Inc. Chicago, IL, USA) for Windows. Continuous variables were shown as the mean±standard deviation. Chi-Square test was used to analyze the categorical variables. Student t-test was used for continuous variables. P values <0.05 was considered as statistically significant.
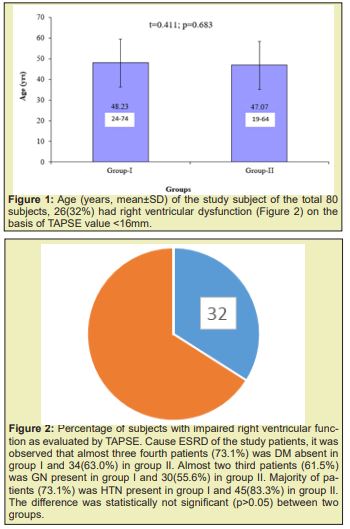
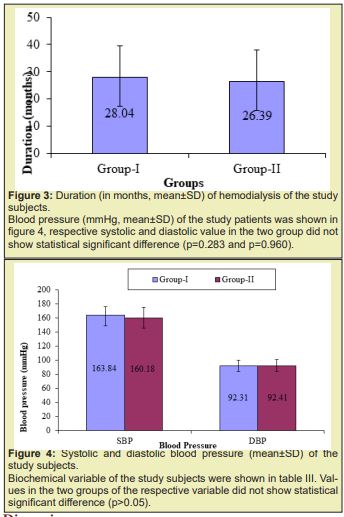
The age distribution of the study patients shown in (Table 1 &2), (Figures 1-4) it was observed that the Mean age was 48.23±11.39 (yrs) with ranged from (24-74 years) in group I and 47.07±12.02 years with ranged from (19-64 years) in group II. More than three fourth (80.8%) patients were male in group I and 37(68.5%) patients were Male in group II. The difference was statistically not significant (p>0.05) between two groups. Results were expressed as numbers (percent) Chi square test was performed to calculate statistical association. p<0.05 was taken as level of significance. Cause ESRD of the study patients, it was observed that almost three fourth patients (73.1%) was DM absent in group I and 34(63.0%) in group II. Almost two third patients (61.5%) was GN present in group I and 30(55.6%) in group II. Majority of patients (73.1%) was HTN present in group I and 45(83.3%) in group II. The difference was statistically not significant (p>0.05) between two groups. Blood pressure (mmHg, mean±SD) of the study patients was shown in, respective systolic and diastolic value in the two group did not show statistical significant difference (p=0.283 and p=0.960). Biochemical variable of the study subjects were shown in Table 3. Values in the two groups of the respective variable did not show statistical significant difference (p>0.05). Results were expressed as mean±SD and range (maximum-minimum). Student’s unpaired t-test was performed to calculate statistical difference. p<0.05 was taken as level of significance. Arteriovenous fistula flow of the study subjects was shown in Table 4. Arteriovenous fistula blood flow in group I (1603±382ml/min) was significantly higher than group II (792±189ml/min) (p=0.001). Results were expressed as mean±SD and range (minimum-maximum). Student unpaired t-test was performed to calculate statistical difference. p<0.05 was taken as level of significance. Distribution of the study subjects on the basis of site of AVFs was shown in Table 4. In group 1. 14 (53.8%) out if 26 had brachiocephalic fistula and rest 12 (46.2%) radio cephalic fistula. In Group II 51(94.4%) out of 54 had radio cephalic fistula. Results were expressed as numbers (percent) Chi square test was performed to calculate statistical association. p<0.05 was taken as level of significance. Relationship between AVFs flow and TAPSE was shown in Figure 4. AVFs flow showed significant inverse relationship (r=-0.431, p=0.001) with TAPSE in all study subjects.




Cardiovascular disease is the leading cause of the death in patients receiving chronic renal replacement therapy18. Arteriovenous fistulas have superior longevity, lower infection and mortality rates and are associated with lower cost, and hence have become the vascular access of choice for patients needing dialysis.21 Despite their association with a lower mortality, AVFs have significant effects on cardiac functions predominantly related to the increase in preload and cardiac output. It should be emphasized, at the outset, that determining the exact effects of AVFs on cardiac functions is fraught with problems for a couple of reasons: patients with end stage renal disease (ESRD) requiring dialysis almost invariably have volume overload due to water and salt retention. They also have pressure load due to arterial sclerosis and hypertension, and increased cardiac output secondary to chronic anemia. In addition, many hemodialysis patients have significant pre-existing myocardial, valvar or coronary heart disease. It is, therefore, often difficult to tease out the exact contribution of an AVF to cardiac dysfunction in hemodialysis patients. Nevertheless, worsening in cardiac functions soon after AVF creation has been observed favoring a causative effect of the AVF on certain cardiac functions. The current literature suggests that the creation of AVF can cause or exacerbate the following conditions: congestive heart failure, left ventricular hypertrophy, pulmonary hypertension, right ventricular dysfunction, coronary artery disease, and valvar dysfunction.21
AVFs and congestive heart failure
Congestive heart failure (CHF) is highly prevalent among patients with ESRD. Approximately 35–40% of patients with ESRD have an established CHF diagnosis at initiation of hemodialysis.22,23,24 Patients with ESRD and CHF have a far worse prognosis than those without CHF.5,25 The creation of an AVF leads to shunting of blood flow from the high resistance arterial system into the low resistance venous system, with a subsequent rise in venous return and cardiac output 24. Second, the presence of an AVF decreases arterial impedance and thus lessens the left ventricular after load. The lowering of arterial impendence may also reduce the effective circulating volume of the systemic circulation, activating arterial baroreceptors, and leading to secondary increase in cardiac sympathetic tone, contractility, and cardiac output.26,27,28 The net effect of AVFs is a significant increase in cardiac output.
These studies consistently showed an increase in LV end-diastolic dimension (LVEDd), contractility, stroke volume and cardiac output within 7–10 days after the surgical construction of AVF. 29,27,30 On average, the creation of an AVF increases cardiac output by 15–20% and left ventricular end-diastolic pressure by 5-10%.28,31 Additionally, biomarkers secreted in response to hypervolemia such as atrial naturistic peptide (ANP)and brain naturistic peptide (BNP), are both substantially increased29,27 suggesting the presence of an cardiac volume overload despite an optimal overall body volume status. The impact of these physiological effects of AVF on the cardiac function is controversial. While many studies suggested that the decreased vascular resistance and the increased cardiac output are predisposing factors for the development or the worsening of heart failure.24 others suggested that the decrease in peripheral resistance and blood pressure with a parallel increase in ejection fraction could be potentially beneficial.32
AVFs and right ventricular dysfunction
A few recent studies examined the effect of AVFs on echocardiographic parameters of RV dysfunction. DiLullo,14 demonstrated that AVFs were associated with impaired RV systolic function (assessed by tricuspid annular plane excursion–TAPSE) and significant RV chamber dilatation compared to those dialyzed via central venous catheters. Paneni,19 studied the prevalence of RV dysfunction in 94 patients on hemodialysis and 26 patients on PD. In this study, Tissue Doppler-derived myocardial performance index (MPI) was used as an indicator of global right ventricle function. Right ventricular dysfunction was more prevalent in hemodialysis patients compared with PD patients (71.3vs. 34.6%). The prevalence of right ventricular dysfunction further increased in patients with brachial AVF compared with the radial access (90.6% vs. 61.3%). In another study of 41 Hemodialysis patients with AVFs, RV dysfunction was present in 18 patients (44%).33 In keeping with the findings by Paneni17,19 the presence of AVF was associated with RV dysfunction independent of pulmonary artery pressure value. The presence of right ventricular dysfunction independent of pulmonary artery pressure values, argues against a major role for pulmonary hypertension in the development of RV dysfunction in ESRD patients19,33 It also suggests that AVF-dependent volume overload may by itself play a major role in triggering RV dysfunction in patients undergoing hemodialysis.19
TAPSE: The systolic movement of the lateral wall of the right ventricle allows to register one of the most obvious movements in a normal echocardiography. The TAPSE is a measure of the distance that the tricuspid annulus travels during systole along the longitudinal plane.15 its use as an indicator of systolic function of the right ventricle was proposed since 198432 and subsequent publications have shown an adequate correlation with nuclear ventriculography34 and magnetic resonance.15
Advantages: TAPSE is simple, less dependent on optimal image quality, and reproducible, and it does not require sophisticated equipment or prolonged image analysis. Disadvantages: TAPSE assumes that the displacement of a single segment represents the function of a complex 3D structure. Furthermore, it is angle dependent, and there are no large-scale validation studies. Finally, TAPSE may be load dependent1.5 Miller,35 published a study of 183 patients treated for steal and high flow using the MILLER banding technique. A total of 114 patients presented with hand ischemia (steal) and 69 patients presented with clinical manifestations of pathologic high access flow such as congestive heart failure. Overall, 183 patients underwent a combined 229 bandings with technical success achieved in 225. Complete symptomatic relief (clinical success) was attained in 109 steal patients and in all high-flow patients. The average follow-up time was 11 months, with a 6-month primary band patency of 75% and 85% for steal and high-flow patients, respectively. At 24 months the secondary access patency was 90% and the thrombotic event rates for upper-arm fistulas, forearm fistulas, and grafts were 0.21, 0.10, and 0.92 per access year, respectively. In a few instances, banding may not be the best option, such as AVFs with a greater than 20-mm peri-anastomotic area. In these cases, a piece of vein, or expanded polytetrafluoroethylene, can be used to move the inflow to a more distal location. The revision using distal inflow (RUDI) technique involves ligation of the fistula at its origin followed byre-establishment of the fistula via bypass from a more distal arterial source to the venous limb of the AVF. Its design adds resistance to the system because it uses a smaller distal artery as inflow and lengthens the fistula with a smaller diameter bypass. Nevertheless, revascularization surgeries are complex and met with various degrees of success.36 Hemodialysis appeared to patient’s choice over peritoneal dialysis owing to its advantages like the dialysis is performed by nurses, treatment time is shorter, patients can socialize with staff and other patients, and they receive continuous follow-up evaluation by a medical team.37
Hemodialysis attributed to cardiac dysfunction mainly accounted by derangement in vascular flow. Of the two commonly practiced fistula brachiocephalic and radio cephalic; brachiocephalic fistula found to have flow rate above 1000ml/min. This high flow rate shown to have linked to increase risk of right ventricular dysfunction17which is so far evaluated by right ventricular fractional area change (RVFAC), tissue Doppler imaging (TDI), right ventricular ejection fraction (RVEF). RVFAC required better image quality.38. It is important to make sure that the entire right ventricle is in the view, including the apex and the lateral wall in both systole and diastole. Care must be taken to exclude trabecula ions while tracing the right ventricular area.17 Two dimensionally (2D) derived estimation of RVEF is not recommended, because of the heterogeneity of methods and the numerous geometric assumption.17Tissue Doppler imaging (TDI) is less reproducible and is angle dependent, there are limited data in all ranges and in both sexes.17
There is tremendous improvement in echocardiography starting from detection of valvar stenosis, regurgitation, assessing cardiac function. TAPSE is a method to measure the distance of systolic excursion of the RV annular segment along its longitudinal plane, from a standard apical 4-chamber window. It is inferred that the greater the descent of the base in systole, the better the right ventricular systolic function. TAPSE is usually acquired by placing an M-mode cursor through the tricuspid annulus and measuring the amount of longitudinal motion of the annulus at peak systole.17 TAPSE correlated strongly with radionuclide angiography, with low inter observer variability 32. It has also been validated against right ventricular ejection fraction (RVEF) and RV fractional area shortening (RVFAC).35,39 There are, however, few number of studies which evaluated TAPSE in hemodialysis patients. This study evaluated the relationship of TAPSE and arteriovenous fistula blood flow. Brachiocephalic and radio cephalic fistula show to have difference in blood flow 500-800 mL/min in the first, in excess of 1000 mL/min in the second10. This difference found to have right ventricular dysfunction which is more common in brachiocephalic fistula. Paneni,19 showed right ventricular function were found to be significantly decreased in hemodialysis patients, particularly in those with a brachial AVF rather than radial AVF. In this study, 53.8% patient had brachiocephalic fistula than radio cephalic fistula in group of impaired right ventricular function (53.8% vs 46.2%). Recent study subjects had right ventricular dysfunction was 32.4% as evidenced by TAPSE value <16mm. Momtaz40 in their study found value to be 28%. However, Yilmaz41 in their study reported that 34.09% subject had TAPSE value <16mm which justify the present value of 32.4 % at the present study. Pulmonary hypertension as one of the reasons for development of right ventricular dysfunction may be excluded considering the study which demonstrated that right ventricular is significantly higher in those with hemodialysis compared to peritoneal dialysis when pulmonary hypertension adjusted as a confounding factor.33
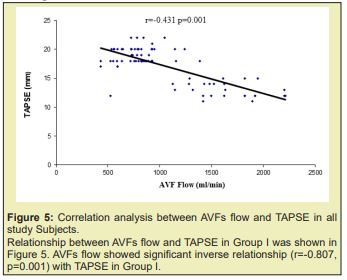
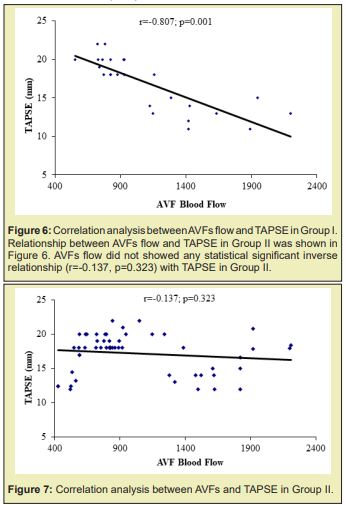
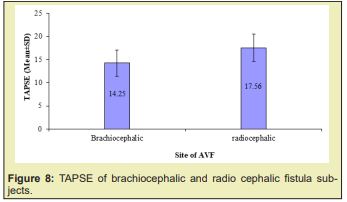
Yilmaz41 in their study demonstrated that AVF flow was high in those reduce right ventricular function group compare to normal right ventricular function group (1631.53±738.17ml/min vs 1060.55±539.92ml/min). In the present study also demonstrated similar trend in this regard, those developed right ventricular dysfunction presented with blood flow about 1603±381ml/min; compared to 791.52±188ml/min in those without right ventricular dysfunction Table 5 & Figures 5-8 supporting the proposition at possible detrimental effect of arteriovenous blood flow in development of right ventricular function. Significant high blood flow in those with brachiocephalic arteriovenous fistula was observed compared to radio cephalic arteriovenous fistula. The former group also had lower TAPSE value compared to the counterpart. These finding of the present study provided strong data support to believe that impaired right ventricular function in end stage renal disease patients on maintenance hemodialysis possibly due to high blood flow in arteriovenous fistula and alarming us to be concerned about with patients in long term care.42
High AVF flow and right ventricular dysfunction should be consider as an important risk factor during evaluation of cardiac complication in chronic hemodialysis patient. Further appropriately controlled studies need to be performed in order to definitively assess the issue. From this study it can be said that increasing vascular access (AVFs) flow lead to impairment of right ventricular function which may increase morbidity and mortality of patients.
Approved by ethical review committee, Dhaka Medical College Hospital, Dhaka, Bangladesh
Taken from each patients enrolled.
It was a single center study with a relatively small sample size.
None.
None.
None.
- 1. Brescia MJ, Cimino JE, Appel K, et al. Chronic hemodialysis using venepuncture and a surgically created arteriovenous fistula. New England Journal of Medicine. 1966;275(20):1089–1092.
- 2. Vassalotti JA, Jennings WC, Beathard GA, et al. Fistula First Breakthrough Initiative: Targeting Catheter Last in Fistula First. Semin Dial. 2012;25(3):303–310.
- 3. Piazza G, Goldhaber SZ. The Acutely Decompensated Right Ventricle. Chest. 2005;128(3):1836–1852.
- 4. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology and epidemiology and prevention. Circulation. 2003;108(17):2154–2169.
- 5. Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999;10(7):1606–1615.
- 6. Tonelli M, Wiebe N, Knoll G, et al. Systematic Review: Kidney Transplantation Compared With Dialysis in Clinically Relevant Outcomes. Am J Transplant. 2011;11(10):2093–2109.
- 7. Foley RN, Parfrey PS, Harnett JD, et al, Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47(7):186–192.
- 8. Ramapriya sinnakirouchenan , Jean L Holley, peritoneal dialysis versus hemodialysis: risk, benefit and access issue. Adv Chronic Kidney Dis. 2011;189160:428–432.
- 9. Haas G. Versuche der Blutauswaschung am Lebenden mit Hilfe der Dialyse. Klinische Wochenschrift. 1925;4(1):13–14.
- 10. Kolff W, Berk H, Welle NM, et al. The Artificial Kidney: a dialyser with a great area. Acta Medica Scandinavica. 1944;117(2):121–134.
- 11. Quinton W, Dillard D, Scribner BH, Cannulation of blood vessels for prolonged hemodialysis. Trans Am Soc Artif Intern Organs. 1960;6:104–113.
- 12. Davidson I, Chan D, Dolmatch B, et al. Duplex ultrasound evaluation for dialysis access selection and maintenance: a practical guide. J Vasc Access. 2008;9:1–9.
- 13. Field M, MacNamara K, Bailey G, et al. Primary patency rates of AV fistulas and the effect of patient variables. J Vasc Access. 2008;9(1):45–50.
- 14. Di Lullo L, Floccari F, Polito P, Right ventricular diastolic function in dialysis patients could be affected by vascular access. Nephron Clin Pract. 2011;118:257–261.
- 15. Kjaergaard, Dilek Akkan, Kasper Karmark Iversen, et al. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur J Heart Fail. 2007;9(6-7): 610–166.
- 16. Zhao LJ, SM Huang, T Liang, et al. Pulmonary hypertension and right ventricular dysfunction in hemodialysis patients, Eur Rev Med Pharmacol Sci. 2014;18(21):3267–3273.
- 17. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713.
- 18. Malík J, Tuka V, Mokrejsová M, et al. Mechanisms of chronic heart failure development in end stage renal disease patients on chronic hemodialysis. Physiol Res. 2009;58(5):613–621.
- 19. Paneni F, Gregori M, Ciavarella GM, et al. Right ventricular dysfunction in patients with end stage renal disease. Am J Nephrol.2010;32(5):432–438.
- 20. Advantages and pitfalls. Clinical Journal of American Society Nephrology.2(5):1043–1053.
- 21. Mohamad Alkhoulia, Paul Sandhub, Khlaed Boobesc, et al. Cardiac complications of arteriovenous fistulas in patients with end-stage renal disease. Nefrologia. 2015;35(3):234–245.
- 22. Levin A, Foley RN. Lok CE, Fistula first initiative cardiovascular disease in chronic renal insufficiency. American Journal of Kidney Disease. 2000;36(6):24–30.
- 23. Harnett JD, Foley RN, Kent GM, et al. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int. 1995;47(3):884–890.
- 24. Levin A, Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. Semin Dial. 2003;16(2):101–105.
- 25. Stack AG, Bloehmbergen WE. A cross-sectional study of the prevalence and clinical correlates of congestive heart failure among incident US dialysis patients. Am J Kidney Dis. 2001;38(5):992–1000.
- 26. Bolignano D, Rastelli S, Agarwal R, et al. Pulmonary hypertension in CKD. American Journal of Kidney Disease. 2013;61(4):612–622.
- 27. Ori Y, Korzets A, Katz M, et al. Hemodialysis arteriovenous access a prospective haemodynamic evaluation. Nephrol Dial Transplant. 1996;11(1):94–97.
- 28. London GM, Left ventricular alterations and end-stage renal disease. Nephrol Dial Transplant. 17(l);2002:29–36.
- 29. Iwashima Y, Horio T, Takami Y, et al. Effects of the creation of arteriovenous fistula for hemodialysis on cardiac function and natriuretic peptide levels in CRF. Am J Kidney Dis. 2002;40(5):974–982.
- 30. Savage MT, Ferro CJ, Sassano A, et al. The impact of arteriovenous fistula formation on central hemodynamic pressures in chronic renal failure patients: a prospective study. Am J Kidney Dis. 2002;40(4):753–759.
- 31. Movilli E, Viola BF, Brunori G, et al. Long-term effects of arteriovenous fistula closure on echocardiographic functional and structural findings in hemodialysis patients: a prospective study. Am J Kidney Dis. 2010;55(4):682–689.
- 32. Korsheed S, Eldehni MT, John SG, et al. Effects of arteriovenous fistula formation on arterial stiffness and cardiovascular performance and function. Nephrology Dialysis Transplantation. 2011;26(10):3296–3302.
- 33. Said K, Hassan M, Baligh E, et al. Ventricular function in patients with end-stage renal disease starting dialysis therapy: a tissue Doppler imaging study. Echocardiography. 2012;29(9):1054–1059.
- 34. Ueti OM, Camargo EE, Ueti Ade A, et al. Assessment of right ventricular function with Doppler echocardiographic indices derived from tricuspid annular motion: comparison with radionuclide angiography. Heart. 2002;88(3):244–248.
- 35. Miller GA, Goel N, Friedman A, et al. The MILLER banding procedure is an effective method for treating dialysis-associated steal syndrome. Kidney Int. 2010;77(4):359–366.
- 36. Minion DJ, Moore E, Endean E. Revision using distal inflow: a novel approach to dialysis-associated steal syndrome. Ann Vasc Surg. 2005;19(5):625–628.
- 37. Dimkovic N, Oreopoulos D. Management of Elderly Patients With End-Stage Kidney Disease. Semin Nephrol. 2009;29(6):643–649.
- 38. Justin Z Lee, See Wei Low, Ahmed K Pasha, et al. Comparison of tricuspid annular plane systolic excursion with fractional area change for the evaluation of right ventricular systolic function: a metaanalysis. Open Heart. 2018;5(1):1–7.
- 39. Lopez Candales A, Dohi K, Rajagopalan N, et al. Defining normal variables of right ventricular size and function in pulmonary hypertension: an echocardiographic study. Postgrad Med J. 2008;84(987):40–45.
- 40. Mohamed Momtaz, Hussein Al Fishawya, Ula Mabid Aljarhia, et al. Right ventricular dysfunction in patients with end-stage renal disease on regular hemodialysis. The Egyptian Society of Internal Medicine. 2013;25:127–132.
- 41. Yilmaz S, Yetim M, Yilmaz BK, et al. High hemodialysis vascular access flow and impaired right ventricular function in chronic hemodialysis patients. Indian Journal of Nephrology. 2016;26(5):352–556.
- 42. Kaul S, Tei C, Hopkins JM, Assessment of right ventricular function using two-dimensional echocardiography. American Heart Journal. 1984;107:526–531.

