The work had as objective to evaluate the effect of the extract of fruit of murtilla in the metabolism of wistar rats that received a hypercaloric diet and monosodium glutamate. In the first step, monosodium glutamate was injected into the animals and, in the third the murtilla extract was administered by gavage. The polyphenolic compounds and the antioxidant activity of the extracts and, in the in vivo tests, the weight gain were evaluated; feed intake and apparent digestibility; liver lipid content; the amount of adipose tissue; and the fatty acid profiles of these tissues were analyzed. Due to the fact that the extract of the berry fruit has a greater variety of antioxidant compounds, we hypothesized that it would be beneficial for the treatment of obesity. However, when associated with a hypercaloric diet and monosodium glutamate, the hepatic lipid content and the amount of epididymal adipose tissue increased (p<0.05). The groups fed hypercaloric diets (E1 and E2) showed an increase in weight gain, apparent digestibility and amount of adipose tissue and a reduction in food intake. Both the liver and the adipose tissue of the groups fed with a hypercaloric diet showed higher levels of monounsaturated fatty acids and lower levels of polyunsaturated fatty acids. These results suggest that murtilla extract in association with a hypercaloric diet is not suitable as a therapy for the treatment of obesity and its complications.
Keywords: Dietary fat, Lipid metabolism, Fatty acids analysis, Obesity
Obesity is a multifactorial disease and among its main causes is the imbalance between food intake and caloric expenditure.1 This pathology drives the development of changes in the organism’s vital functions, including the endocrine, pulmonary, cardiac and immune systems.2 The excessive consumption of lipids and carbohydrates are risk factors for obesity and cardiovascular diseases.3 Obesity can be induced in animals via isolated cafeteria diet consumption,4 used due to the high concentrations of simple sugars and saturated fats,5 or in combination with monosodium glutamate applications.6
The cafeteria diet offered to animals is usually composed of unhealthy food that are part of the human diet7 such as bacon, lard, condensed milk, soft drinks, chocolate, nuts, roasted peanuts and various types of sweets.3,8 Generally, these diets produce an approximately 30 to 40% increase in total body weight of the animals at the end of 12 weeks9 or sometimes inducing changes in glycemia and lipid metabolism (serum or tissue) without significantly increasing body weight.10
Studies with extracts or flours produced from plants have been performed with animal models, aiming to reduce body weight or the amount of adipose tissue. Interest in murtilla (Ugnimolinae Turcz) has increased in the food and pharmaceutical industry, mainly due to its high content of phenolic compounds,11 which can act in the prevention and treatment of various diseases, including obesity, metabolic syndromes, neurodegenerative diseases, and cancers.12
Murtilla, also known as uñior murta, is a plant originally from Chile, and its fruits are used in the preparation of jellies, liqueurs, syrups, juices and chocolates.13,14 Murtilla fruits present bioactive compounds that help to promote health,15 with emphasis on antioxidant activity due to its content of flavonols and polyphenols.11,16-18 The therapeutic use of it is indigenous in origin and based on its aromatic and adrenergic properties (i.e., from tannins and flavonoids) that are important in the treatment of some health disorders.18,19 Therefore, due to the antioxidant action of the murtilla, was hypothesized that it has a beneficial effect in the prevention and treatment of obesity, therefore, the aim of this study was to evaluate the effect of the murtilla extract on metabolism wistar rats that received a hypercaloric diet and monosodium glutamate.
Preparation of hydroalcoholic extracts from murtilla fruits
Murtilla fruits were obtained from cultivated plants. The cultivated fruits (14-4 genotype) were grown in the germoplasm bank of the experimental station of the Instituto de Investigaciones Agropecuarias (INIA), Puerto Saavedra (38°, 45’S, 73°, 21’W) La Araucania, Chile. The cultivated fruits were harvested in April 2011 and dried using an industrial drying cabinet (1.8m long, 2.2m high, 3.5m deep) at a constant temperature of 70°C for six hours to a final moisture content of 5.4%. The fruits were vacum packed and protected from light and oxygen. Then, the dehydrated fruits were grounded, and 1.0g of sample was placed in a 250mL erlenmeyer flask with 40mL of ethanol 49.5% (v/v ethanol/water). After that, the Erlenmeyer was kept at 30°C for 50 minutes under agitation in a water bath. The extract was centrifuged at 5000rpm for 15 minutes, filtered using a Whatman no2 filter paper and stored under refrigeration at 7°C in amber flasks until analysis.
Total phenolic content and antioxidant activity determined by DPPH method
The total phenolic content of the murtilla fruit extract was determined in triplicate according to the Folin-Ciocalteau spectrophotometric method.19 The extract was diluted in the mixture of ethanol: water at the concentration of 1:10 (v/v). An aliquot of 0.5mL of diluted extract was transferred to a test tube, and 2.5mL of the Folin-Ciocalteau: water solution (1:10,v/v) was added. The mixture was vortexed and rested at room temperature for five minutes. Then, 2.0mL of the sodium carbonate 4%(m/v) solution was added and the mixture was agitated again and rested for two hours at room temperature and protected from the light. Absorbance was read at 740nm using a UV-1203 spectrophotometer (Shimadzu Corporation, Japan). The results were calculated using a standard curve of gallic acid with known concentrations (2.5 to 50µg/mL), and they were expressed as mg of gallic acid (GAE)/g of dehydrated fruit.
The antioxidant activity was determined following the DPPH radical (1,1-diphenyl-2-picrylhydrazyl)20 using Trolox (6-hydroxy-2 5 7 8-tetramethylchroman-2-carboxylic acid) (0.1µmol) as a standard to construct the calibration curve. A solution composed of 0.5mL of extract diluted in an ethanolic solution 80%, 3.0mL of ethanol 99%, and 0.3mL of the DPPH radical 0.5mM diluted in an ethanol: water solution (80:20 v/v) was added to a test tube. A blank sample was prepared by substituting the extract volume for an equal volume of ethanol 99%. The tubes were agitated and incubated for 45 minutes at room temperature and protected from light. The absorptivity reading was performed using a spectrophotometer (Shimadzu, model UV 1203) at 515nm. The antioxidant activity results were expressed as µmol TEAC/gof dehydrated fruit.
Animal protocol and diets
Male rats (Rattusnorvegicus), wistar strain, were obtained from the Central Biotério of the Federal University of Viçosa with an initial weight of 232.49±30.61g. The animals were kept in individual cages at 21°C with a 12-hour light/dark cycle (6am at 6pm) and receiving ad libitum commercial rations and mineral water. The total experiment lasted 12 weeks and was separated into three phases, as shown in Figure 1.
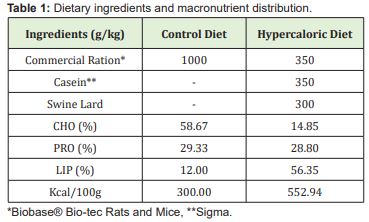
The control animals (C1 and C2) were fed commercial feed pellets (Biobase® Bio-tec Rats and Mice - 300.00 kcal/100g; 58.67% of carbohydrates, 29.33% of proteins and 12.00% of lipids), while animals in the E1 and E2 groups consumed a hypercaloric diet composed of 35g ground commercial feed (Biobase® Bio-tec Rats and Mice), 35g casein (Sigma Aldrich®) and 30g lard (552.94 kcal/100g, where 14.85% of carbohydrates, 28.80% of proteins and 56.35% of lipids) Table 1.In step 1, C1 and E1 groups were submitted to subcutaneous injections of 12.5% saline with 125mg/mL of solution at a dose of 1.25mg/g body weight.21 C2 and E2 rats received subcutaneous injections of monosodium glutamate (Ajinomoto®) at a 24% concentration, with 240mg/mL of solution in the posterior cervical region once a week at a dose of 4mg/g of body weight. For both groups, the volume of solution injected weekly was calculated, with 50% of total volume applied on Monday and 50% on Friday.
At step 2, the animals ate only commercial pellet feed or a hypercaloric diet. At step 3, the animals remained in their respective groups receiving commercial feed in the form of pellets and the hypercaloric diet, and an extract of murtilla was administered once a week via gavage to groups that received monosodium glutamate (C2 and E2), while mineral water was administered to those who received the saline solution in step 1 (C1 and E1). The same dosage of saline or monosodium glutamate was used (i.e., for each gram weight of the injected rat, 0.0157 mL of extract of murtilla or mineral water was administered).
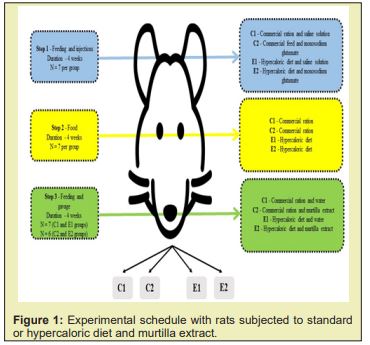
Morphometric parameters, food consumption and apparent digestibility
The body weight and naso-anal distance were measured weekly. From these data, the
Body Mass Index (BMI) = [body weight (g) / length2 (cm)]4 and the Lee Index (LI) = [body weight cube root (g) / naso-anal size (cm)]22 were calculated.
The feed intake was calculated weekly based on the difference between the quantity of food offered and the quantity left uneaten.4 Last week, feces were collected to calculate the diet’s apparent digestibility. Based on total food intake and feces eliminated, the nutrients absorbed by the digestive tract were calculated according to the equation:
apparent digestibility = (quantity absorbed / quantity ingested) x 100.
Sacrifice of animals and preparation of samples
At the end of the experiment, the rats were submitted to general anesthesia with Propofol subcutaneously into the flank region at 10mL/kg of body weight. After deep anesthesia, the animal was placed in the supine position and a gauze soaked with 70% alcohol was passed over the length of it. A thumb was used to feel along the animal's trunk to find the spot where the xiphoid cartilage ended. A needle at an angle of 45° was introduced with a slope to the left. Approximately 4mL of blood was collected by cardiac puncture that promoted death by exsanguination under anesthesia. The liver and epididymal and visceral tissues were removed for necropsy and weighed. Means ± standard deviation followed by different letters in the same line are significantly different using Tukey’s test (p <0.05).
Determination of total lipids and fatty acids profile of liver and adipose tissue
After dehydration of the liver and adipose tissue for 24h at 40°C in an oven with air circulation, total lipids were determined in triplicate (mg/g of tissue).23 The epididymal and visceral adipose tissues were weighed for the extraction of lipids. The extracted lipids were dissolved in 1mL hexane to determine the fatty acids. The obtained samples were saponified with sodium hydroxide in methanol (0.5M) and methylated with a solution of ammonium chloride, methanol and sulfuric acid.24 Hexane (5mL) was added to the sample, which was stirred for 10 seconds. From the supernatant was removed a 3mL aliquot, which was concentrated with nitrogen gas, resuspended in 100mL hexane, and used for analysis of the fatty acid profile by gas chromatography.
The fatty acid profile was determined by high-performance gas chromatography using a gas chromatograph (HP 5890), equipped with a Supelco capillary column SP 2560, 100mx0.25mm, df 0.20μm coupled to a flame ionization detector. The temperature setting was 130°C (1.0min) at 170°C (6.5°C/min), 170 to 215°C (2.75 °C/min), 215°C (12min), 215 to 230°C (40°C/min), 230°C (6min). The injector and detector temperatures were 270 and 280°C, respectively. The samples (0.3ml) were injected using the direct injection technique. The fatty acids of 6, 8, 10, 12, 14, 15, 16 (cis and trans), 17, 18 (cis and trans), 20, 22 and 24 carbon atoms, saturated and unsaturated, were identified by comparison to the gas chromatograph data of authentic methylated standards eluted under the same conditions.
The percentage obtained was achieved by total normalization of the areas obtained from the chromatogram using the following equation: %N = (Actr/ΣActotal)x100; where: Actr = area obtained from the fatty acid at the retention time; and Actotal = total area of the chromatogram obtained.
Statistical analysis
A one-way analysis of variance was performed to evaluate the results, and the differences between the obtained mean values were examined by Tukey’s test at 5% probability, using IBM SPSS (Statistical Package for the Social Sciences, version 22.0).
Compliance with ethics requirements
All national and institutional guidelines for the care and use of Laboratory Animals were followed. This experiment was conducted according to the Ethical Principles in Animal Experimentation from Brazil, according to Law 11.794, of October 8, 2008.25 The project has been approved by the Ethics Committee on Animal Use of Federal University of Viçosa, Brazil (Protocol no 111/2013).
Total phenolic content and antioxidant activity
The total phenolic content of the murtilla extract was 30.35±1.20mg GAE/g of dehydrated fruit, while the antioxidant activity was 239.37±4.75µmol TEAC/g of dehydrated fruit with a percent inhibition of the radical of 47.98%.
Morphometric parameters
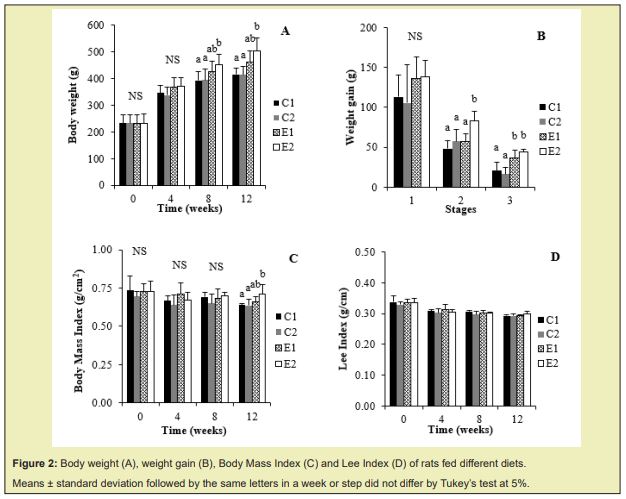
Until week 4 (step 1) there was no significant difference between groups in body weight and weight gain Figure 2A and 2B; only at week 8 (end of step 2) the group that received the hypercaloric diet and monosodium glutamate (E2) presented greater weight gain than the other groups Figure 2B. At the end of the experiment (week 12), the E1 and E2 groups did not differ statistically in body weight and weight gain.
For Body Mass Index, only at the end of the experiment (week 12), the E2 group that received the murtilla extract, showed a statistically difference than C1 and C2 groups Figure 2C. There was no significant difference between the groups considering the Lee Index Figure 2D, demonstrating that the murtilla extract did not alter this parameter.
Measures of food consumption and apparent digestibility
In all steps, food consumption in the control diets (C1 and C2) was significantly higher than the other groups Figure 3A, demonstrating that monosodium glutamate (Step 1) and murtilla did not lead to higher food consumption than their respective controls receiving saline and water, respectively. However, the apparent digestibility of control diets (C1 and C2) was significantly lower than the other groups Figure 3B in both steps, demonstrating that the animals fed the hypercaloric diet (E1 and E2) present increased intestinal absorption of nutrients.
Determination of total lipids and fatty acids profile of liver
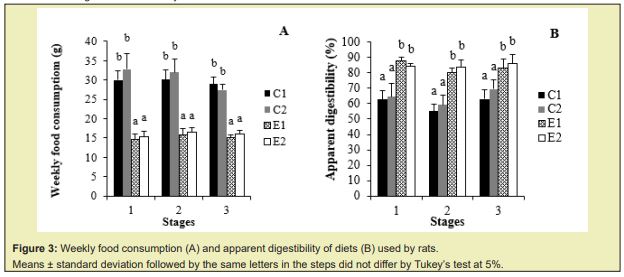
The group that received the hypercaloric diet and the murtilla extract (E2) presented a higher liver weight and lipid content in this organ than the C1 group, demonstrating that this extract in association with a hypercaloric diet increased the lipid content in the liver Figure 4A, which was represented by monounsaturated fatty acids Table 2.
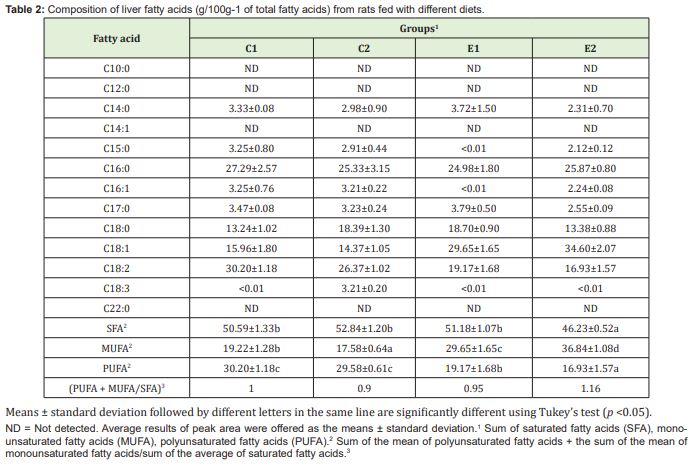
The groups fed with a hypercaloric diet (E1 and E2) had a higher hepatic content of monounsaturated fatty acids and lower polyunsaturated fatty acids than the control groups (C1 and C2). Among the essential fatty acids, linoleic acid had the highest hepatic concentration Table 2, and only the C2 group had the highest concentration of linolenic fatty acid. The groups presented similar values for the hepatic ratio of [Polyunsaturated Fatty Acids (PUFA) + Monounsaturated Fatty Acids (MUFA)]/ Saturated Fatty Acids (SFA) Table 2.
Determination of total amount of adipose tissue and its fatty acids profile
The groups fed with a hypercaloric diet (E1 and E2) presented more epididymal and visceral adipose tissue than the control groups (C1 and C2), demonstrating that murtilla extract in association with a hypercaloric diet increased the amount of epididymal adipose tissue Figure 5 but did not differ for visceral adipose tissue when compared to the E1 group.
The C1, C2 and E1 groups presented a higher concentration of saturated fatty acids in visceral adipose tissue compared to epididymal adipose tissue. The groups fed with a hypercaloric diet (E1 and E2) presented higher amounts of monounsaturated and lower polyunsaturated fatty acids, in fatty tissue and liver, than the control groups (C1 and C2). Among the essential fatty acids, linoleic acid had a higher concentration in the adipose tissues Table 3. Means ± standard deviation followed by different letters in the same line are significantly different using Tukey’s test (p <0.05). The E1 group showed the lowest values for the PUFA + MUFA/SFA ratio in both tissues evaluated Table 3 due to higher concentration of saturated fatty acids of these tissues.
In view of the antioxidant capacity of the murtilla and its content of phenolic compounds, it was expected that it would help to combat reactive oxygen species and play a beneficial action in the treatment of obesity. However, the expected result was not achieved, instead of helping to improve the prognosis of obesity, the murtilla extract associated with a hypercaloric diet and monosodium glutamate intensified obesity through aspects such as increased epididymal adipose tissue and content hepatic lipid. Antioxidant activity is a consequence of synergism between different phenolic compounds and cannot be attributed specifically to a single constituent. The total phenolic content and antioxidant activity of murtilla fruit extract can be explained by the presence of several molecules with significant biological activity such as quercetin, epicatechin, gallic, benzoic and hydro caffeic acids which have been identified previously in other study.11
The results obtained from phenolic content and antioxidant activity corresponds to evaluated fresh blackberry (Rubuscoesins), raspberry (Rubusideaus), strawberry (Fragariavesca) and blueberry (Vacciniummyrtilus) samples, observed values of total phenolic content like 3.55mg GAE/g; 1.78mg GAE/g; 2.44mg GAE/g and 6.70mg GAE/g, respectively.26 In another study, which also analyzed the phenolic content in murtilla extract using the Folin Ciocalteu method, the result was a phenolic content, variable according to the solvent used, between 23-34 GAE/mg.27
According to the results observed in the induction of obesity, in the first four weeks (Step 1), monosodium glutamate did not promote changes in body weight and weight gain in animals. The group fed a high-calorie diet and receiving monosodium glutamate (E2) in step 1 showed greater weight gain than the other groups during step 2. And in step 3 the murtilla extract promoted greater weight gain in the animals.
At step 3, the E2 group presented a higher Body Mass Index (p<0.05) than the control groups (C1 and C2). There was no significant difference in Lee Index between the groups during the study, demonstrating that murtilla extract did not alter this parameter.
During the three stages of the experiment, food consumption in the groups that received commercial rations (C1 and C2) was significantly higher, demonstrating that the receivement of monosodium glutamate (Step 1) and myrtle extract (Step 3) associated with a non-hypercaloric diet led to a higher food consumption when compared to controls that received saline and water, respectively. In rats, the low food intake of hypercaloric and hyperlipidic diets has been associated with greater satiety promoted by slow gastric emptying.28 However, it has been shown that rats fed with a hypercaloric diet have higher food consumption resulting in increased body weight and adipose tissue accumulation,29 because these diets usually provide hyperphagia due to palatability, which can lead to changes in the feeding behavior of animals.30,31
When wistar rats were fed with a hypercaloric diet, a lower food consumption, higher final body weight, Body Mass Index and body fat content were observed compared to the control group. At all steps, the apparent digestibility of the groups that received the commercial ration (C1 and C2) was significantly lower than that of other groups, showing that the groups fed with a hypercaloric diet (E1 and E2) had higher intestinal absorption of nutrients.
The application of monosodium glutamate to animals in this study promoted the hepatic lipogenesis only in the group that received hypercaloric diet and murtilla extract (E2), since findings from groups C1 and C2 did not differ. In a study carried out in rats with a control group and a group with the application of monosodium glutamate, without the inclusion of hypercaloric diets, it was possible to observe an increase in the rate of obesity and lipid deposits.32 In another study, the parenteral administration of monosodium glutamate resulted in the development of some metabolic alterations such as obesity, hyperglycemia, and hyperlipidemia.33
Only C1 and E2 groups differed in liver weight, whereas animals from E2 group (hypercaloric diet and murtilla extract) presented higher lipid levels in this organ, differing from a previous study34 that showed no significant difference in liver weight between control group and hypercaloric diet group based on commercial ration, corn oil and condensed milk. Studies with animals fed with a cafeteria diet or hypercaloric diet showed more fat deposited in the liver, increasing its total weight compared to those fed with a control diet.35 Higher hepatic weight observed in coffee-fed animals resulted from their lipid content.36
Based on the ratio (PUFA+MUFA)/SFA, E1 group presented the lowest values for the ratio (PUFA + MUFA)/SFA in both tissues evaluated Table 3. The majority of adipose tissues (epididymal and visceral) in animals fed with a hypercaloric diet (E1 and E2) compared to control groups (C1 and C2) demonstrated that this kind of diet can promote an increase in the amount of adipose tissue. A significant increase in epididymal and retroperitoneal adipose tissues was observed in rats submitted to hypercaloric diets.37 Rats fed with a hypercaloric diet or cafeteria diet showed greater weight gain and increased total adipose tissue when compared to those fed with a standard diet.38,39
The intake of cafeteria diets with excess of macronutrients such as lipids and carbohydrates can result in accumulation of energy as fat, especially to visceral and to a lesser extent in subcutaneous deposits.40 It was possible to affirm that several changes in wistar rats fed hypercaloric diets are related to the increased abdominal adiposity (visceral) and the obesity seen after long-term adherence to this type of diet.41,42
Notably, intra-abdominal adipose tissue, characterized by body fat around the visceral organs, is associated with negative health effects,43 like dyslipidemia, hypertension, cardiovascular diseases and type 2 diabetes,44 due to its inflammatory action, in which there is an imbalance in the release of pro-inflammatory and anti-inflammatory molecules.2
The C1, C2, and E1 groups had higher concentrations of saturated fatty acids in visceral adipose tissue, and adiposity in this region is directly related to cardiovascular diseases, insulin resistance, hypertriglyceridemia, decreased HDL cholesterol and increased small and dense LDL cholesterol particles.45,46
Animals fed with hypercaloric diets (E1 and E2) presented higher concentrations of monounsaturated fatty acids in adipose epididymal and visceral tissues; the group that received murtilla extract was statistically superior to other groups. According to polyunsaturated fatty acids results, E1 and E2 groups had the lowest concentrations of linoleic and linolenic acid.
The best indicator of obesity in rats, fed with a hypercaloric diet, is body fat since weight increases by approximately 10%, and fat increases 30 to 50%. The accumulation of adipose tissue is associated with several markers of obesity such as weight gain, Body Mass Index and Lee Index.47
The murtilla extract presented a considering antioxidant potential in lipidic food. However, when associated with hypercaloric diet, the extract could promote greater deposition of epididymal adipose tissue than other groups studied.
It is known that phenolic compounds, in addition to antioxidant activity, may exhibit desirable physiological properties such as antiallergenic, antiatherogenic, anti-inflammatory, antimicrobial, antithrombotic, cardioprotective and vasodilating activity.47,48 Although, the main effect of these compounds are related to their antioxidant action, which in addition to help positively in the body's antioxidant defense and reducing the development of degenerative diseases,12,49 should be attributed to foods with potential control of oxidation reactions,47 which can be used to increase the shelf life of hyperlipidic foods.50
Through the benefits of alternative medicine and natural claim, populations have increased consumption of products and/or preparations with high doses of natural antioxidants based on their therapeutic properties. However, it is necessary to perform controlled clinical trials to demonstrate their efficacy and potential toxicity under specific conditions, since this has not yet been explored.51
While some epidemiological evidence shows a positive correlation between the consumption of natural antioxidants and health, observational and case-control studies commonly present conflicting results, such as increased risk of cardiovascular disease and cancer, when such compounds was used in suboptimal concentrations. Some explanations for these results are a lack of information on dose/response effects in humans, the bioavailability of antioxidants in the diet and individual physiological responses.52
Murtillafruit extract presents a wide variety of antioxidant compounds with high antioxidant potential. However, when associated with a hypercaloric diet and monosodium glutamate, it increased the content of hepatic lipids and the amount of epididymal adipose tissue. The groups fed with a hypercaloric diets increased in weight gain, apparent digestibility, amount of adipose tissue and decreased food consumption. In both liver and adipose tissues, groups fed with a hypercaloric diet showed a higher concentration of monounsaturated fatty acids and lower concentration of polyunsaturated fatty acids.
Murtilla extract, in association with a hypercaloric diet and monosodium glutamate, did not present as a promising therapy for the treatment of obesity and its complications. However, it may promote other health benefits due to its antioxidant compounds and may potentiate the effect of diet hypercaloric and monosodium glutamate in induction of obesity in rats.
Support provided by Project FONDEF AF10I1007 from CONICYT, Chile and to CAPES, Brazil, for the scholarship granted. This study was reviewed for English for Jacqueline Barakat.
No support was provided.
The first author, Beatriz Barakat, was responsible for the development of the work, being guided by the authors Martha Elisa Ferreira de Almeida, José Antônio de Souza Cruz Ramos and Erick Scheuermann. The authors Thalita Riquelme Augusto Obara and Richtier Gonçalves da Cruz, performed the analysis of the fatty acid profile.
Author declares that there is no conflict of interest.
- 1. Ellison B, Prescott MP. Examining nutrition and food waste trade offs using an obesity prevention context. J Nutr Educ Behav. 2021;53:434–444.
- 2. Barakat B, Almeida MEF. Biochemical and immunological changes in obesity. Arch Biochem Biophys. 2021;708.
- 3. Almeida MEF, Santos VS, Simão AA, et al. Cafeteria diet with chocolate, peanut and cookie: effectiveness in induction of overweight and dyslipidemia in rats. SaBios Rev Saude Biol. 2015;10:15–24.
- 4. Almeida MEF, Simão AA, Corrêa AD, et al. Improvement of physiological parameters of rats subjected to hypercaloric diet, with the use of Pereskiagrandifolia (Cactaceae) leaf flour. Obes Res Clin Pract. 2016;10:701–709.
- 5. Urrutia MAD, Ramos AG, Menegusso RB, et al. Effects of supplementation with kombucha and green banana flour on wistar rats fed with a cafeteria diet. Heliyon. 2021.
- 6. Konrad SP, Farah V, Rodrigues B, et al. Monosodium glutamate neonatal treatment induces cardiovascular autonomic function changes in rodents. Clinics. 2012;67:1209–1214.
- 7. Lalanza JF, Snoeren EMS. The cafeteria diet: A standardized protocol and its effects on behavior. NeurosciBiobehav Rev. 2021;122:92–119.
- 8. Cesaretti MLR, Kohlmann Junior O. Experimental models of insulin resistance and obesity: lessons learned. Arq Bras Endocrinol Metab. 2006;50:190–197.
- 9. Paula RB, Silva AA, Hall JE. Aldosterone antagonism attenuates obesity–induce hypertension and glomerular hyperfiltration. Hypertension. 2004;43:41–47.
- 10. Naderali EK, Pickavance LC, Wilding JPH, et al. Diet–induced endothelial dysfunction in the rats is independent of the degree of increase in total body weight. Clin Sci. 2001;100:635–641.
- 11. Augusto TR, Salinas ESS, Alencar SM, et al. Phenolic compounds and antioxidant activity of hydroalcoholic extracts of wild and cultivated murtilla (UgnimolinaeTurcz.).Food Sci Technol. 2014;34:667–673.
- 12. Arruda HS, Neri Numa IA, Kido LA, et al. Recent advances and possibilities for the use of plant phenolic compounds to manage ageing–related diseases. J Funct Foods. 2020;75.
- 13. Avello M, Pastene E. Actividad antioxidante de infusos de UgnimolinaeTurcz (“murtilla”).Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 2005;4:33–39.
- 14. Arancibia Radich J, González Blázquez R, Alcalá M, et al. Beneficial effects of murtilla extract and madecassic acid on insulin sensitivity and endothelial function in a model of diet induced obesity. Sci Rep. 2019;9.
- 15. Zura Bravo L, Rodríguez A, Stucken K, et al. Nutritional and organoleptic properties of murta (UgnimolinaeTurcz) berries impregnated with Lactobacillus casei var. rhamnosus and dehydrated by different methods. Food Chem. 2019;299.
- 16. Junqueira Gonçalves MP, Yáñez L, Morales C, et al. Isolation and characterization of phenolic compounds and anthocyanins from Murta (UgnimolinaeTurcz.) fruits. Assessment of antioxidant and antibacterial activity. Molecules. 2015;20:5698–5713.
- 17. Jofré I, Pezoa C, Cuevas M, et al. Antioxidant and vasodilator activity of UgnimolinaeTurcz. (Murtilla) and its modulatory mechanism in hypotensive response. Oxid Med Cell Longev. 2016;1–11.
- 18. Augusto Obara TR, Pirce F, Scheuermann E, et al. Antioxidant activity and sensory analysis of murtilla (UgnimolinaeTurcz.) fruit extracts in an oil model system. Grasas y Aceites. 2017;68:e183.
- 19. Singleton VL, Orthofer R, Lamuela Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin ciocalteau reagent. Methods Enzymol. 1999;299:152–178.
- 20. Brand Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30.
- 21. Novelli ELB, Diniz YS, Galhardi CM, et al. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007;41:111–119.
- 22. Bernardis LL, Patterson BD. Correlation between ‘Lee index’ and carcass fat content in weanling and adult female rats with hypothalamic lesions. J Endocrinol. 1968;40:527–528.
- 23. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J BiochemPhysiol. 1959;37:911–917.
- 24. Hartman L, Lago RCA. Rapid preparation of fatty acids methyl esters from lipids. Lab Pract. 1973;22:475–476.
- 25. Brazil. Lei no 11.794, de 8 de outubro de 2008. Procedures for scientific use of animals. 2008.
- 26. Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Univ Chem Techn Metall. 2005;40:255–260.
- 27. Dicastillo CL, Bustos F, Valenzuela X, et al. Chilean berry Ugnimolinae Turcz. fruit and leaves extracts with interesting antioxidant, antimicrobial and tyrosinase inhibitory properties. Food Res Int. 2017;102:119–128.
- 28. Shivaprasad HN, Gopalakrishna S, Mariyanna B, et al. Effect of Coleus forskohlii extract on cafeteria diet–induced obesity in rats. Pharmacognosy Res. 2014;6:42–45.
- 29. Eguchi R, Cheik NC, Oyama LM, et al. Effects of the chronic exercise on the circulating concentration of leptin and ghrelin in rats with diet induced obesity. Rev Bras Med Esporte. 2008;14:182–187.
- 30. Colombo G, Bazzo ML, Nogueira CL, et al. A study on the short term effect of cafeteria diet and pioglitazone on insulin resistance and serum levels of adiponectin and ghrelin. Braz J Med Biol Res. 2012;45:935–941.
- 31. Gual Grau A, Guirro M, Mayneris Perxachs J, et al. Impact of different hypercaloric diets on obesity features in rats: a metagenomics and metabolomics integrative approach. J Nutr Biochem. 2019;71:122–131.
- 32. Majewski M, Jurgońsk A, Fotschki B, et al. The toxic effects of monosodium glutamate (MSG) – The involvement of nitric oxide, prostanoids and potassium channels in the reactivity of thoracic arteries in MSG–obese rats. Toxicol Appl Pharmacol. 2018;359:62–69.
- 33. Bautista RJH, Mahmoud AM, Königsberg M, et al. Obesity: Pathophysiology, monosodium glutamate induced model and anti obesity medicinal plants. Biomed Pharmacother. 2019;111:503–516.
- 34. Choi KM, Lee YS, Shin DM, et al. Green tomato extract attenuates high fat diet induced obesity through activation of the AMPK pathway in C57BL/6 mice. The J Nutr Biochem. 2013;24:335–342.
- 35. Longo L, Souza VEG, Stein DJ, et al.Transcranial direct current stimulation (tDCS) has beneficial effects on liver lipid accumulation and hepatic inflammatory parameters in obese rats. Sci Rep. 2021;11.
- 36. Reynés B, García Ruiz E, Díaz Rúa R, et al. Reversion to a control balanced diet is able to restore body weight and to recover altered metabolic parameters in adult rats long–term fed on a cafeteria diet. Food Res Int. 2014;64:839–848.
- 37. Duarte ACGO, Fonseca DF, Manzoni MSJ, et al. High fat diet and secretory capacity of insulin in rats. Rev Nutr. 2006;19:341–348.
- 38. Arbo BD, Niches G, Zanini P, et al. Aging affects the response of female rats to a hypercaloric diet. Exp Gerontol. 2018;101:7–12.
- 39. Bonfim THF, Tavares RL, Vasconcelos MHA, et al. Potentially obesogenic diets alter metabolic and neuro behavioural parameters in wistar rats: a comparison between two dietary models. J Affect Disord. 2021;15:451–461.
- 40. Marosti AR, Almeida FN, Moraes SMF, et al. Effects of the cafeteria diet on the salivary glands of trained and sedentary wistar rats. Acta Scient Biol Sci. 2012;34:113–118.
- 41. Nascimento AF, Sugizaki MM, Leopoldo AS, et al. A hypercaloric pellet diet cycle induces obesity and co morbidities in wistar rats. Arq Bras Endocrinol Metab. 2008;52:968–974.
- 42. Metwally FM, Rashad H, Mahmoud AA, et al. Diminishes visceral adiposity, insulin resistance, behavioral alterations via regulation of gene expression of leptin, resistin and adiponectin in rats fed a high cholesterol diet. Physiol Behav. 2019;201:1–11.
- 43. Costa CAS, Alves EG, Gonzalez GP, et al. Computed tomography in the evaluation of abdominal fat distribution associated with a hyperlipidic diet in previously undernourished rats. Radiol Bras. 2007;40:337–340.
- 44. Jeyakumar SM, Reddy MRG, Garlapati C, et al. Diabetogenic diet–induced insulin resistance associates with lipid droplet proteins and adipose tissue secretome, but not with sexual dimorphic adipose tissue fat accumulation in wistar rats. Biochem Biophys Rep. 2020;24.
- 45. Garaulet M, Pérez Llamas F, Pérez Ayala M, et al. Site specific differences in the fatty acid composition of abdominal tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr. 2001;74:585–591.
- 46. Reijrink M, Boer SA, Spoor DS, et al. Visceral adipose tissue volume is associated with premature atherosclerosis in early type 2 diabetes mellitus independent of traditional risk factors. Atherosclerosis. 2019;290:87–93.
- 47. Balasundram N, Sundram K, Samman S. Phenolic compounds in plant and agri–industrial by–products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203.
- 48. Ávila Román J, Soliz Rueda JR, Bravo FI, et al. Phenolic compounds and biological rhythms: Who takes the lead? Trends Food Sci Technol. 2021;113:77–85.
- 49. Sarker U, Oba S. Phenolic profiles and antioxidant activities in selected drought–tolerant leafy vegetable amaranth. Sci Rep. 2020;10.
- 50. Durán RM, Padilla RB. Antioxidantactivity of phenoliccompounds. Grasas Aceites. 1993;44:101–106.
- 51. Galati G, O‘Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemo preventive and anticancer properties. Free Radic Biol Med. 2004;37:287–303.
- 52. Costa NMB, Rosa COB. Alimentos funcionais: componentes bioativos e efeitos fisiológicos. Rio de Janeiro: Rubio; 2010.

