Background: Conjunctival chemosis, a complication of lower blepharoplasty can cause persistent discomfort and functional disturbances with worsening in the postoperative period following surgery.
Methods: A review of the records of the lower blepharoplasty procedures carried out at the Humanitas Research Hospital, Rozzano, Milan, Italy was performed. Patients were categorized into 2 groups depending on the procedure perfomed: (1) transconjunctival blepharoplasty with removal of the fatty lodges with canthopexy and (2) transcutaneous blepharoplasty with removal of the fatty lodges with lateral canthoplasty. Each group was further divided into two more groups based on the surgical method used, that is either (a) cold blade and disposable cautery or (b) radiofrequency cut and coagulation and colorado tip (respectively 1a, 1b, 2a and 2b). All patients underwent a postoperative follow-up up to 24 months, evaluating cosmetic appearance, eyelid scarring and the severity of chemosis. The aim of the study was to investigate which of the surgical procedure causes the lower incidence of persistent type-3 conjunctival chemosis.
Results: 1047 patients who underwent lower lid blepharoplasty were included in the study. 512 patients underwent transcutaneous blepharoplasty and 535 transconjunctival procedure. Among the first group of patients, 266 belong to group 1a and 246 to group 1b. In the second group, 264 were categorized as group 2a and 271 as group 2b. The incidence of type-3 chemosis in the transcutaneous blepharoplasty procedure with lateral canthoplasty was statistically significantly higher than in the transconjunctival approach, considering both the cold blade and the radiofrequency (respectively p=0.012, 0.010, 0.006, 0.004).
Conclusion: Persistent type-3 conjunctival chemosis has a higher incidence when associated with lateral canthus surgery and with the use of radiofrequency.
Keywords: Blepharoplasty, Chemosis, Oculoplastics, Eyelid disease, Eyelid surgery
Lower lid blepharoplasty, a surgical procedure aimed at rejuvenating the appearance of the lower eyelid region/cheek complex, has undergone significant advancements over the years.1 The periorbital region demands meticulous surgical techniques to achieve both aesthetic and functional outcomes.2 The lower eyelid region is characterized by complex anatomical structures, including the orbicularis oculi muscle, the orbital septum, the retaining ligaments, and the underlying fat compartments. Disruption of any of these structures can lead to functional and aesthetic complications.3
Traditional lower lid blepharoplasty techniques, such as the transcutaneous and transconjunctival approaches, have served as the foundation for periorbital rejuvenation. The transcutaneous approach involves an external incision made along the lower eyelid margin, allowing for direct visualization and excision of excess skin and fat. Conversely, the transconjunctival approach utilizes a conjunctival incision to access and reposition orbital fat while avoiding visible external scars. The choice between these two approaches depends on the patient's specific needs and anatomy. Transcutaneous blepharoplasty may be preferred for patients with significant skin laxity or when skin removal is necessary. Transconjunctival blepharoplasty, on the other hand, is suitable for those primarily concerned with fat pad removal and minimal skin excess.4 Ultimately, the decision should be made through a careful assessment of the patient's individual goals and anatomical characteristics to achieve the best aesthetic outcome. Moreover, the surgery of the lower lid can be associated to surgical interventions targeting the canthal region of the eye, in order to reshape the lateral canthus. Canthoplasty is a more invasive procedure that involves the manipulation and repositioning of the lateral canthal tendon, typically by detaching and reattaching it to a new location on the orbital bone. This method allows for a significant alteration of the eyelid's shape or position.5 Conversely, canthopexis is a less invasive technique that aims to provide support and stability to the canthal structures without tendon repositioning. It involves reinforcing the existing attachments of the lateral canthus, often utilizing sutures or grafts to augment the support without significantly altering the position.6
Complications can range from minor and transient to more severe and lasting, underscoring the importance of surgical precision and patient selection. Among these, one of the minor complications of this surgical treatment and in particular of lower blepharoplasty is acute or chronic conjunctival chemosis.7
Chemosis is defined as transudative edema of the bulbar conjunctiva and/or fornix that creates a space between the conjunctiva and Tenon's capsule and is characterized by visible elevation of the conjunctival tissue with an underlying straw-colored fluid collection.8
In the literature, the incidence is approximately 1%, but chemosis has become a growing problem with more recent advances in surgical technique that is, from simple traditional skin straightening and tightening in blepharoplasty and face lifts to more comprehensive procedures involving lateral canthal support and orbicularis surgery and published complication rates of chemosis persisting over 2 or 3 weeks went from 1% to 34.5%.9,10
The purpose of this study is to understand which surgical procedure has the lower incidence of persistent conjunctival chemosis.
A retrospective review of the medical records of lower blepharoplasty procedures was performed. Patients who underwent primary blepharoplasty with correction of the lateral canthus with cantoplasty or canthopexy were included in the study. We excluded patients affected by dysthyroidism, conjunctivocalasis and with a history of allergy or atopia. The study adhered to the guidelines of the Health Insurance Portability and Accountability Act and aligned with the principles set forth in the Declaration of Helsinki (2013). Patient consent was secured for the publication of medical photographs featured in the article. Furthermore, the Institutional Review Board granted approval for the study.
All the patients included were divided into 2 groups as follows:
- 1. The first group who underwent transconjunctival procedure with removal of the fatty lodges with canthopexy
- 2. The second group who underwent transcutaneous blepharoplasty with removal of the fatty lodges with lateral canthoplasty.
Each group was further classified on the basis of the instrumentation and material used:
- a. Cold blade and disposable cautery + fibrillar tabotamp and cold-water irrigation
- b. Cutting and coagulation with radiofrequency (Surgitron Dual Frequency 120 Watts, Ell- man International Inc., Hewlett, NY, U.S.A - a 4.00 MHz, high frequency/low temperature radiosurgical unit. Cut mode -power: 30–35- with the A-8 needle for incision and cut- coagulation mode -power: 35–40- with the Empire needle for skin (muscle) flap dissection and excision) and cold-water irrigation.
All the surgeries were carried out under supervision of the same experienced surgeon. The procedures were done under local anesthesia with local injection of lidocaine with epinephrine at 1:100,000 concentration in the place of incision and mebipuvacaine for a regional block. After heart and peripheral oximetry were monitored, the patient was sedated with the slow intravenous injection of 2ml of fentanyl chlorhydrate and 3mg of midazolam. 10% betadine was used for skin disinfection and 5% for cleansing the conjunctival arches for 3 minutes. During the procedure the ocular surface was irrigated with isotonic solution and the cornea protected with viscoelastics.
The technique performed in transcutaneous lower blepharoplasty involves the execution of a sub ciliary skin incision 2mm below the eyelash line, from which the underlying structures were reached. Then, a lower eyelid cutaneous muscle flap with release of the orbito-malar ligament followed by fat removal or transposition and cantoplasty to correct tarsus ligamentous laxity were carried out Figure 1. The process includes incising the orbicularis oculi muscle, separating the lateral canthal tendon from the bone (cantholysis), and reducing the length of the lateral canthal tendon. Subsequently, the tarsus is manipulated to cover and reconnect with the lateral orbital rim at the desired elevation. The incisions were closed with separated 6–0 nylon sutures, which were removed after 7 days.
In transconjunctival blepharoplasty the placement of the transconjunctival incision is approximately 8mm below the lid margin and allowing for a transconjunctival access to the orbital fat. No suturing of the conjunctival incision was performed and lateral canthopexy was performed instead to avoid the so called “round eye” and to correct the lid length to compensate for the vertical volume reduction inherent in fat removal. An opening was made in the orbital septum, starting from the inner part of the lateral orbital bone aligning with the outer corners of the eyes, exposing the canthal tendon. Above and below the tendon, a passage was carefully crafted to enable the passage of a Prolene 4-0 suture needle underneath it. After adjusting the eye's shape and retraction to the preferred degree using the suture, it will be secured to the periosteum of the upper lateral section of the outer orbital wall.
Postoperative care included intensive corneal lubrication with eye protection, eye drops, and ointment.
All patients underwent postoperative follow-up at 7, 15 days and 1,3,6, 9, 12 and 24 months. During the assessment the classification of chemosis proposed by AB Weifeld.11 Table 1 was used to classify the results. In detail:
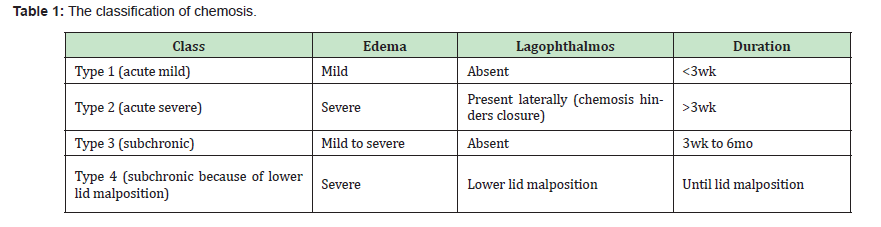
Type 1: Chemosis is a mild form of acute chemosis. A distinguishing feature is the ability to close the eyelid completely.
Type 2: Chemosis represents severe acute chemosis. The swollen and injected conjunctiva protrudes from the palpebral fissure, producing a mass effect. The end result is chemosis-induced lagophthalmos.
Type 3: Chemosis represents subchronic and persistent edema and inflammation of the conjunctiva lasting up to 6 months. The cause of type 3 chemosis is probably related to prolonged lymphatic dysfunction caused by inadequate division and lack of reanastomosis of lymphatic channels within the eyelids and surrounding facial tissues. Lagophthalmos is generally not present.
Type 4: Chemosis represents chemosis caused by malposition of the lower eyelid and/or ectropion. It is important to distinguish the associated incomplete lid closure in type 4 from lagophthalmos caused by mass effect of severe chemosis in type 2. In type 4, both edema and inflammation are present and are the result of conjunctival exposure.Type 4 chemosis will not resolve until the lower eyelid malposition and ectropion are corrected.
For the statistical analysis StataSE 18 software (StataSE, USA) was used. A chi-square test was performed to measure the significant differences among the four groups. The level of significance was set at p< 0.05 and measured between groups 1a and 1b, 2a and 2b, 1a and 2a, 1b and 2b.
1047 patients who underwent lower lid blepharoplasty between 2005 and 2023 at the Department of Ophthalmology, Humanitas Research Hospital, Rozzano, Milan, Italy were included in the study. The majority of patients, 785 (74.9%) were female, while 262 (25.1%) were male. The total mean age was 58.3±8.7 years old, the females was 54.7±9.4 and the males was 69.7±5.4. 512 patients underwent transcutaneous blepharoplasty with lateral canthoplasty and 535 transconjunctival procedure with canthopexy. Among the first group of patients, 266 received treatment with cold blade and disposable cautery and 246 with radiofrequency cut and coagulation and colorado tip. In the second group, 264 were treated with the cold blade technique and 271 with the radiofrequency technique Figure 2. The average time taken to perform a bilateral procedure was 48 minutes for transconjunctival and 56 for transcutaneous.
The characteristics of the sample were summarized in Table 2.
The mean follow-up time was 27.8±2.3 months. The main outcome was to measure the incidence of persistent type 3 conjunctival chemosis following different techniques of lower eyelid blepharoplasty and different materials and methods to perform them.
The incidence of postoperative conjunctival chemosis, considering the four groups of patients is summarized in Table 3.
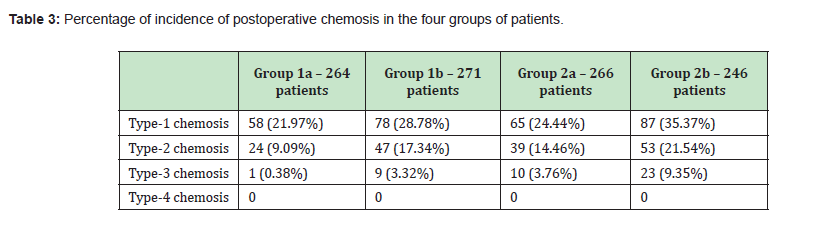
In Table 4 are displayed the p-values resulting from the analysis of the coupling of the 4 groups.
There were no major postoperative complications. We did not find some of the complications reported in the literature, such as: alterations of eyelid closure, lagophthalmos, epicanthal, eyelid ptosis, eyebrow drooping, senile orbit and diplopia.12,13
Conjunctival chemosis is defined as transudative edema of the bulbar conjunctiva and/or fornix and is characterized by visible swelling of the conjunctiva. It is a collection of fluid in the plane between the bulbar conjunctiva and the tenon's capsule overlying the sclera. Signs and symptoms of conjunctival chemosis are epiphora, foreign body sensation due to exposure of the conjunctiva and de-epithelialization due to exposure with hyperemia and slight visual changes due to maldistribution of the tear film.114-16
In general, chemosis is not a highly debilitating complication, it can cause epiphora, irritation, slight alterations in visual acuity due to alteration of the distribution of the tear film. The irregularity of the ocular surface can paradoxically cause both epiphora and corneal dryness, as the normal lamination of the tear film and its drainage is interrupted. This can certainly decrease patient satisfaction with the aesthetic outcome of a successful surgery. In most cases, chemosis resolves spontaneously early in the postoperative period, but for some patient’s resolution is delayed.
The aim of our study was to study whether a difference in the incidence of type-3 chemosis exists among 4 groups of patients treated with different surgical techniques.
The main result of our study was a statistically significant difference in the incidence of type-3 chemosis, with a higher incidence in transcutaneous blepharoplasty with correction of the position of the lateral canthus than blepharoplasty performed transconjunctivally and a higher incidence when radiofrequency is used.
The pathophysiology of conjunctival chemosis after eyelid surgery is multifactorial and is typically a combination of inflammation, increased permeability of the conjunctival vessels, venous congestion, and impaired lymphatic drainage. Consequently, chemosis is supposed to be caused by irritation due to the disinfection protocol, intraoperative conjunctival exposure, reduced eyelid tone in the postoperative period, and ineffective lymphatic drainage due to dissection and healing.17,18
Paradoxically with the advances in surgical techniques related to blepharoplasty and upper third face lift which combine the treatment of lateral canthal support and dissection of the orbicularis muscle and maintaining ligament division and straightening for the upper half of the cheek, rates of published complications of chemosis persisting beyond 2 or 3 weeks increased from 1% to 34.5%.19,20
Type-1 chemosis is typically accompanied by inflammation of the conjunctiva, indicating that extravasation is due to increased vascular permeability and venous engorgement. Our findings are consistent with the pathogenesis of type-1 chemosis, as no statistically significant difference was found among the four groups, except for the comparison between group 2a and 2b.
On the contrary, type-3 chemosis is not supported by inflammatory processes, the conjunctiva is not hyperemic and this indicates the failure of lymphatic drainage as the main cause.21,22
The disinfection protocol with polyvinylpyrrolidone-iodine (PVP-I), a broad-spectrum biocompatible antimicrobial, is pointed out as inducing conjunctival inflammation in the perioperative period, but this molecule is used in daily clinical practice without significant adverse events. It is known in the literature that concentrations equal to or lower than 0.5% of PVP-I are practically non-irritating even if administered up to six times a day. This protocol is used routinely in bulb surgery where there is no evidence of conjunctival chemosis and this leads to the exclusion of antiseptic treatment as a cause of chemosis.23,24
The timing of surgery may induce proportional postoperative chemosis. Surgery time becomes longer when multiple procedures are combined. This may be due to long periods of exposure and dehydration with conjunctival and corneal de-epithelialization. This consideration is mentioned in the literature, but not supported with specific studies.11,13
The use of radiofrequency compared to the scalpel was evaluated in relation to the tissue damage and healing induced by the two surgical instruments because they may be involved in the manifestation of type-3 chemosis caused by the inflammation resulting from surgical trauma and the direct interruption of drainage lymphatic or indirect due to scarring of the anatomical region treated.
There are few studies that have considered these data and in all of them no significant difference was found between radiofrequency and scalpel incision in lower blepharoplasty with regards to inflammation, intratissue hemorrhage, recovery of sensitivity and formation of the scar.25 Our results are in line with the literature.26,27
The common factor in these procedures was deep surgery in the lateral canthal region (canthotomy and canthopexy) which can interrupt lymphatic drainage.
A superficial collecting lymphatic system of the eyelids in the lateral region has been described for many years.9,10,28 The superficial system can be at risk of harm during incisions and dissection in the infraorbital area, while the deep system may be vulnerable to damage during dissection around the orbicularis retaining ligament and the zygomatic-cutaneous ligaments. Hence, harm to both the superficial and deep lymphatic systems, particularly on the lateral aspect, could potentially contribute to postoperative chemosis and persistent edema.29 In most cases, chemosis resolves spontaneously early in the postoperative period, but for some patients, resolution is delayed. There are numerous treatment options for conjunctival chemosis including both conservative and surgical management.17,30
In 2016, it was defined as a deep system that drains the conjunctiva and eyelids.29 Although a deep collecting lymphatic system had been suggested by previous histological and ink injection studies.31-35
The authors found that the lower eyelid and conjunctiva are drained by interconnecting the superficial and deep lymphatic systems of the face. The superficial system is vulnerable to damage in incisions and dissections in the infraorbital area. The deep system is vulnerable to damage in dissection around the orbicularis retaining ligament and zygomatic-cutaneous ligaments. It is suggested that concomitant damage to the superficial and deep lymphatic system, especially laterally, may be responsible for chemosis and persistent edema.
Disruption of existing conjunctival and cutaneous lymphatics during surgical dissection and cauterization may cause accumulation of extracellular fluid causing conjunctival chemosis and lymphedema in the skin of the eyelids and subcutaneous tissues. Similarly, severe or prolonged postoperative inflammation can cause scarring of the lymphatic vessels and poor lymphatic drainage of the conjunctiva and eyelids. Although short-lived, postoperative edema resulting in localized venous congestion may also contribute to conjunctival chemosis independent of damage to regional lymphatic ducts. The superficial lymphatic system drains the eyelids and is laterally dominant, as we described previously.10
The deep lymphatic system drains the conjunctiva and connects with the superficial lymphatic system through the preseptal orbicularis muscle. There is a paucity of valves in the lymphatic collecting vessels, similar to the venous system of the head and neck.35
It has been found that the lymphatic pathways draining the face may also parallel the neurovascular system, with medial lymphatics following the facial vein and lateral lymphatics running adjacent to the facial nerve and vessels.
The superficial lymphatic system of the face drains the conjunctiva and the skin, with precollectors (0.05 to 0.1 mm) forming in the dermis near the medial and lateral canthi. This form collecting lymphatic vessels (>0.1 mm) superficial to the preseptal orbicularis muscle at the level of the lateral and medial third of the orbicularis muscle retaining ligament. These collecting lymphatic vessels travel within the subcutaneous fat of the cheek. Each of these vessels remains within a distinct fat compartment, the medial within the nasolabial fat compartment and the lateral within the lateral orbital fat compartment. Lateral and medial collecting systems drained to the preauricular and submandibular nodes, respectively. Furthermore, the superficial system has connections to the deep drainage system, with the precollectors traveling through the dermis and preseptal orbicularis muscle fibers to join the deep system.
The deep facial lymphatic system drains the conjunctiva directly from the precollectors traveling through the tarsal plate and meibomian glands in the lateral third of the lower eyelid. The deep lymphatic system is joined by connections with the superficial lymphatic system from the skin of the eyelid and face as already mentioned.
The deep system lymphatic precollectors then travel beneath the surface of the preseptal orbicularis muscle in the lateral lower quadrant to the junction of the orbicularis muscle retaining ligament and the lateral orbital thickening, traveling through the superficial orbicularis muscle retaining ligament and widening to the vessels collectors that travel in the fat sub-orbicularis oculi in the roof of the prezygomatic space. At the level of the most cranial zygomaticocutaneous ligaments, the collecting vessels descend into the preperiosteal fat, from which the zygomaticus major originates, and then descended below the deep fascia to travel adjacent to the facial nerve to reach the preauricular lymph nodes within the parotid.29
In our study, the incidence of type-3 chemosis was higher in blepharoplasty with transcutaneous technique associated with canthoplasty compared to transconjunctival technique associated with canthopexy. The association between transcutaneous blepharoplasty and canthoplasty, as well as transconjunctival blepharoplasty and canthopexy, lies in their respective surgical approaches and goals.
Canthoplasty, which involves repositioning the canthal tendon and often requires access to deeper structures, is better facilitated through the same external incision utilized in transcutaneous blepharoplasty. In contrast, canthopexy aligns well with the less invasive nature of transconjunctival blepharoplasty, as both approaches involve minimal disruption to the external skin and focus on more conservative modifications. This association allows for a more harmonious and complementary surgical approach in addressing specific concerns of the eyelid region while minimizing potential scarring and preserving tissue integrity based on the depth of the incision.
Of all the factors considered, trauma to the lymphatic drainage system was the one that proved to be significant as demonstrated by our results which show that the cases of chronic conjunctival chemosis were greater in transcutaneous surgery associated with canthoplasty compared to transconjunctival blepharoplasty associated with canthopexy.
The results showed that the lower eyelid blepharoplasty technique with lateral canthus treatment performed by a single surgeon, with the same local and systemic anesthesia, with the use of the same bacteriostatic and the same postoperative therapy has a higher incidence of type-3 chemosis in the transcutaneous technique compared to the transconjunctival one and that in the two groups a and b which differ in the surgical materials used, group a of both techniques had a lower incidence of type-3 chemosis and faster resolution of acute post-surgical chemosis.
It is certain that for the prevention of type-3 chemosis all the favorable factors discussed must be well considered such as the use of bacteriostatics with known indications and abundant irrigation of washing solution, the use of radiofrequency must respect parameters that do not histologically damage the tissues too much, both for cutting and haemostasis. The key point is the 'judicious' dissection of the lateral canthal structures in order to reduce direct or indirect interruption due to edema and fibrotic reaction of the superficial and deep drainage system especially in transcutaneous surgery associated with canthoplasty where the incidence of persistent chemosis is higher in percentage than transconjunctival associated with canthopexy.
“Conceptualization, A.D.M, V.V. and M.K.; methodology, A.D.M, V.V. and M.K.; software, V.F.; validation, P.V. and F.C.; formal analysis, A.D.M.; investigation, G.B.; resources, A.G.; data curation, A.G.; writing—original draft preparation, A.D.M.; writing—review and editing, A.D.M. and V.F.; visualization, G.B.; supervision, P.V. and F.C. All authors have read and agreed to the published version of the manuscript.”
None.
This Research Article received no external funding.
Regarding the publication of this article, the authors declare that they have no conflict of interest.
- 1. Rizk SS, Matarasso A. Lower eyelid blepharoplasty: analysis of indications and the treatment of 100 patients. Plast Reconstr Surg. 2003;111(3):1299-306.
- 2. Neves JC, Arancibia Tagle D, Medel Jiménez R, et al. Periorbital Surgical Anatomy. Facial Plast Surg. 2020;36(3):317-328.
- 3. Kakizaki H, Malhotra R, Madge SN, et al. Lower eyelid anatomy: an update. Ann Plast Surg. 2009;63(3):344-351.
- 4. Andretto Amodeo C, Casasco A, Icaro Cornaglia A, et al. The suborbicularis oculi fat (SOOF) and the fascial planes: has everything already been explained?. JAMA Facial Plast Surg. 2014;16(1):36-41.
- 5. Shorr N, Goldberg RA, Eshaghian B, et al. Lateral canthoplasty. Ophthalmic Plast Reconstr Surg. 2003;19(5):345-352.
- 6. Patel BC, Volner K, Malhotra R. Transconjunctival Blepharoplasty. In StatPearls; StatPearls Publishing: Treasure Island (FL). 2023.
- 7. Zoumalan CI, Roostaeian J. Simplifying Blepharoplasty. Plast Reconstr Surg. 2016;137(1):196e-213e.
- 8. Ní Dhubhghaill S, Faris C. The Management of Chemosis after Blepharoplasty. Facial Plast Surg. 2023;39(1):53-56.
- 9. Pan WR, Suami H, Taylor GI. Lymphatic drainage of the superficial tissues of the head and neck: anatomical study and clinical implications. Plast Reconstr Surg. 2008;121(5):1614-1624.
- 10. Pan WR, Le Roux CM, Briggs CA. Variations in the lymphatic drainage pattern of the head and neck: further anatomic studies and clinical implications. Plast Reconstr Surg. 2011;127(2):611-620.
- 11. Weinfeld AB, Burke R, Codner MA. The comprehensive management of chemosis following cosmetic lower blepharoplasty. Plast Reconstr Surg. 2008;122(2):579-586.
- 12. Morax S, Touitou V. Complications of blepharoplasty. Orbit. 2006;25(4):303-318.
- 13. Patrocinio TG, Loredo BA, Arevalo CE, et al. Complications in blepharoplasty: how to avoid and manage them. Braz J Otorhinolaryngol. 2011;77(3):322-327.
- 14. Principles and Practice of Ophthalmic Plastic and Reconstructive Surgery: Bosniak S (edn), with Nine Section Editors and 140 Contributors. Philadelphia, PA, Saunders. 1996;2:1208. Illustrated. Journal of Oral and Maxillofacial Surgery. 1997:pp.55,533.
- 15. Levine MR, Davies R, Ross J. Chemosis following blepharoplasty: an unusual complication. Ophthalmic Surg. 1994;25(9):593-596.
- 16. Westfall CT, Shore JW, Nunery WR, et al. Operative complications of the transconjunctival inferior fornix approach. Ophthalmology. 1991;98(10):1525-1528.
- 17. Thakker MM, Tarbet KJ, Sires BS. Postoperative chemosis after cosmetic eyelid surgery: surgical management with conjunctivoplasty. Arch Facial Plast Surg. 2005;7(3):185-188.
- 18. Honrado CP, Pastorek NJ. Long-term results of lower-lid suspension blepharoplasty: a 30-year experience. Arch Facial Plast Surg. 2004;6(3):150-154.
- 19. Honrado CP, Pastorek NJ. Long-term results of lower-lid suspension blepharoplasty: a 30-year experience. Arch Facial Plast Surg. 2004;6(3):150-154.
- 20. Prischmann J, Sufyan A, Ting JY, et al. Dry eye symptoms and chemosis following blepharoplasty: a 10-year retrospective review of 892 cases in a single-surgeon series. JAMA Facial Plast Surg. 2013;15(1):39-46.
- 21. McCord CD, Kreymerman P, Nahai F, et al. Management of post blepharoplasty chemosis. Aesthet Surg J. 2013;33(5):654-661.
- 22. Enzer YR, Shorr N. Medical and surgical management of chemosis after blepharoplasty. Ophthalmic Plast Reconstr Surg. 1994;10(1):57-63.
- 23. Day AC, Wormald R, Coronini Cronberg S, et al. Royal College of Ophthalmologists Cataract Surgery Commissioning Guidance Development Group. The Royal College of Ophthalmologists' Cataract Surgery Commissioning Guidance: executive summary. Eye (Lond). 2016;30(3):498-502.
- 24. York KK, Miller S, Gaster RN, et al. Polyvinylpyrrolidone iodine: corneal toxicology and epithelial healing in a rabbit model. J Ocul Pharmacol. 1988;4(4):351-358.
- 25. Kashkouli MB, Kaghazkanai R, Mirzaie AZ, et al. Clinicopathologic comparison of radiofrequency versus scalpel incision for upper blepharoplasty. Ophthalmic Plast Reconstr Surg. 2008;24(6):450-453.
- 26. Silverman EB, Read RW, Boyle CR, et al. Histologic comparison of canine skin biopsies collected using monopolar electrosurgery, CO2 laser, radiowave radiosurgery, skin biopsy punch, and scalpel. Vet Surg. 2007;36(1):50-6.
- 27. Turner RJ, Cohen RA, Voet RL, et al. Analysis of Tissue Margins of Cone Biopsy Specimens Obtained with “Cold Knife,” CO2 and Nd:YAG Lasers and a Radiofrequency Surgical Unit. J Reprod Med. 1992;pp.607–610.
- 28. Pan WR, le Roux CM, Levy SM, et al. The morphology of the human lymphatic vessels in the head and neck. Clin Anat. 2010;23(6):654-661.
- 29. Shoukath S, Taylor GI, Mendelson BC, et al. The Lymphatic Anatomy of the Lower Eyelid and Conjunctiva and Correlation with Postoperative Chemosis and Edema. Plast Reconstr Surg. 2017;139(3):628e-637e.
- 30. Garner and Klintworth’s Pathobiology of Ocular Disease - 3rd (edn). 2023.
- 31. Cook BE Jr, Lucarelli MJ, Lemke BN, et al. Eyelid lymphatics II: a search for drainage patterns in the monkey and correlations with human lymphatics. Ophthalmic Plast Reconstr Surg. 2002;18(2):99-106.
- 32. Guo W, Zhu Y, Yu PK, et al. Quantitative study of the topographic distribution of conjunctival lymphatic vessels in the monkey. Exp Eye Res. 2012;94(1):90-97.
- 33. Gusev AM. Lymph Vessels of Human Conjunctiva. Fed Proc Transl Suppl. 1964;23:1099-1102.
- 34. Collin HB. Ocular lymphatics. Am J Optom Arch Am Acad Optom. 1966;43(2):96-106.
- 35. Taylor GI, Caddy CM, Watterson PA, et al. The venous territories (venosomes) of the human body: experimental study and clinical implications. Plast Reconstr Surg. 1990;86(2):185-213.

