Fukushima nuclear power plant once got impact from an earthquake, and it was disabled by a tsunami following. The chain reaction stopped but the remaining products of the chain reaction still release tremendous energy continuously, and the cooling system doesn’t work. Continuous seawater was injected into the reaction cores to cool down the cores, but radioactive seawater after being used to cool down the cores accumulated. At last, Japan made the decision to eject the nuclear wastewater into the sea, but this would threaten people’s health which is same important as life. The stability of a nucleus should be determined by the number of the neutrons and protons it contained, when the total number of nucleons inside the nucleus was under a limitation, the nucleus could be stable when the N/Z (ratio of the number of neutrons over the number of protons) approaches a value about 1, this value will be larger as the Z grows because the stronger electromagnetic forces will make more β+decay of protons which produce neutron and is in a dynamic equilibrium with β- decay of neutrons which produce proton; therefore, to make up some α particles into the chain reaction products remained in the reaction cores may convert the remained radioactive nuclei therein to be stable ones, and the fusion of α particles with the radioactive nuclei in the reaction cores of which the elemental numbers are all heavier than iron can also absorb the energy that may be released in the processes, applying thin α particles beam could ensure the α particles only to merge with the radioactive nuclei in the reaction cores but never each other.
Keywords: Fukushima nuclear power plant, Nuclear strong interaction, Nuclear decay, Stable nuclide, α particle
In 2013, an earthquake hit the Fukushima power plant. The fission processes were stopped immediately by the power plant; however, when the following tsunami came, the cool-down systems for the reaction cores were disabled, and this caused the problem. The chain reaction stopped, but the remaining nuclear waste is still radioactive, and they release tremendous heat continuously. When the cooling system stopped working, the reaction cores became extremely hot and melted the surrounding things. At last, the report said that seawater was used to cool down the core and the temperature is under control, but this isn’t end.
Now ten years after the Fukushima earthquake, only more and more seawater has been used to cool down the core in these years, but the reaction cores are still decaying and releasing tremendous heat and the used seawater could not be effectively treated. The seawater after being used to cool down the reaction cores which contains high concentration of the radioactive materials from the reaction cores accumulated and they were not dealt effectively, also there is no way to stop the reaction cores from releasing heat by the remaining reactions after the chain reaction was stopped. Japan then decided to eject the radioactive water into the sea, but such action would deeply influence the environment we human live in and reduce the feasible food on the earth especially in the oceans. Is it really impossible to solve the problem? To determine this, we need to study nuclear physics first.
At 1909, after electron was discovered, Rutheford found an atom has a core where contains all its mass while has only 10(-4) times of the magnitude of the atom. The chemical property of an atom is only determined by the charge in its core and the surrounding electrons, but the nucleus has its unique properties determined by the nucleons which it consists of and their interaction. In a nucleus, the nucleons were combined with each other tightly by the nuclear strong force which can overcome the electromagnetic force in the distance of the radius of a nucleus
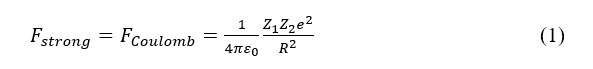
where R=r0A(1/3), is the mass number of the nucleus and r0≈1.2fm experientially, thereby it can be computed that the density of a nucleus is constant, about 2.29412×1014g/cm3, it is in same order of the magnitude of the density of a neutron star. And the nuclear strong force can overcome a potential barrier of
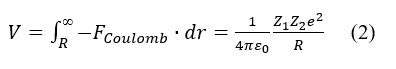
moreover, nuclear strong force is a short-range force different from Coulomb force, it only exists between nearby nucleons in a nucleus (otherwise the density of a nucleus can’t be a constant value), it is just like particles that lock nearby nucleons together to form the entire nucleus but can’t exert in nucleons that are further than nearby ones. Of course the formation of such particle release energy, only with releasing energy could the system have no minus entropy created in such a process. According to the second law of thermodynamics, any nature process can only happen in this direction, unless there is probabilistic factors participated in. In 1935, Yukawa proposed his theory of π meson exchange force theory to explain the force between nucleons; thereby, he gained the Nobel prize in 1949. In fact, any interaction must have its exchanging particle as the medium, since interaction is something that does exist and more than the material that possessed it, and existence is the essence of material according to Marxism.
α, β and γ are three typical types of nuclear decays, their reactions could be written as α decay:
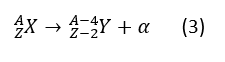
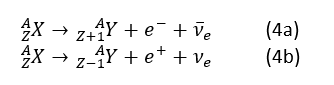
where ν-e and ve are anti-neutrino and neutrino for electron, possibly are the exchanging particles for the interaction between nucleon and electron, like photon is the exchanging particle for the electromagnetic interaction, the acceleration of a charged particle would release photons; therefore, the acceleration of a particle that participated in this interaction would release neutrinos. γ decay is when a nucleus jumping from its excited state to its normal state, photon of high energy would be released. Usually this happens after previous two kinds of decays or any other decay that changes the type of the nuclide, which could provide enough energy to make the new nuclei into its excited states, and release γ ray when it recovered to its ground state.
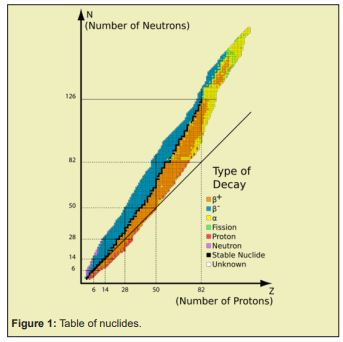
A neutron is always unstable, feels like to get β- decay and then became a proton and an electron; however, when two protons were combined by the nuclear strong force, a proton inside probably will get β+ decay, caused by the strong electromagnetic force which is comparable with the strong nuclear force, and become a neutron again. β+ decay of proton and β- decay of neutron can compensate each other; therefore, in a stable nucleus which consists of many protons and neutrons, the value of the numbers of protons over the number of neutrons will be about 1, and this value can be less if the charge number of this nuclide is larger, because stronger electromagnetic forces from the charges will make the protons get β+ more easily, β- decay of proton and β- decay of neutron in a nucleus should be in an equilibrium. This is consistent with the real distribution of stable nuclides of different numbers of charge (protons), as shown in Figure 1, the proton-neutron ratio of the stable nuclides has a deviation from 1 and became less when the number of protons raised, the electromagnetic force is long range force while nuclear strong force only exists between nearby nucleons, and stronger electromagnetic force promotes β+ of protons to convert them into neutrons. It is also fact that the element would have β- decay when it has more neutrons, while a nucleus with same number of neutrons would have β+ decay when it has more protons, as shown in the figure, this implies that the β- decay of neutron and β+ of proton are in dynamic equilibrium when they are combined with each other by the nuclear strong force within a nucleus, the β- decay of protons and β- decay of neutrons are happening from time to time although they compensate each other to remain the number of protons and neutrons constant in the nucleus. As the number of protons in the nucleus grew, the stronger electromagnetic force will make protons combined by the strong nuclear forces to leave the nucleus; therefore, a nucleus would not be stable anymore and can get α decay when the number of protons is too large. In fact, there is no stable element after Z (the number of protons) exceeds 82, all nuclides have either α decay or the β- decay, this is because the nucleus would be unstable when neutrons were decaying to be protons in the dynamic equilibrium between β+ decay of protons and β- decay of neutrons, then the nucleus would get α decay when Z≥82, and too much neutron would also cause β- decay radioactivity.
In summary, a nucleus consists of neutron and proton, they both are nucleons that have nuclear strong force between each other, by which they were combined together to form nucleus. The nuclear strong force is like particles that locks nearby nucleons together, has interaction only in range between nucleons that are combined nearby each other (potential barrier penetration effect can make this easier) but can’t be more in long range as electromagnetic force. It can be stronger than the electromagnetic force between nucleons which are just as close as their radii when the number of protons in the nucleus is not too high. When the number of protons are too high, the electromagnetic force between protons can be stronger than the nuclear strong force between two nuclei. And there is also interaction between nucleon and electron (positron should be just hole of electron in vacuum), which is exchanged by neutrino and anti-neutrino for positron and electron respectively. It is this interaction makes a neutron unstable and get β- decay to become a proton and an electron. A single neutron can’t stably exist because it will get β- decay with a half-life period about 896s, only when a neutron was combined with one or more protons by nuclear strong force can the number of neutrons remain constant, it is because of the electromagnetic forces between protons can make the protons get β+ decay when they were combined so close in the range of the nuclear strong force, it needs absorb energy to have β+ decay and only electromagnetic force that is comparable with nuclear strong force could provide such strong potential to let it happen. When the neutrons are combined with the protons to form the nucleus by the nuclear strong force, overcoming the strong electromagnetic forces between protons that are just as close as the addition of the radii of two nucleons, the electromagnetic forces would induce the protons which were combined with other protons nearby have β+decay, and this compensates the β- decay of the neutrons if they were combined with protons nearby; therefore, the number of neutrons in a nucleus can remain constant when they were combined with enough number of protons, although this is a dynamic equilibrium. Uncertainty principle doesn’t forbid electron from existing in a nucleon, it just can’t have certain momentum when it had certain position in the nucleon, and this could also account for why the velocity of the electron released from β- decay is random, and of continuous spectrum, the eigenstates of velocity are of continuous spectrum and the electron had no certain velocity before. And as the number of charges in a nucleus grew, the number of protons that the neutrons need to be stable would reduce, because the electromagnetic force of the protons is long range force while the nuclear strong force is short range force, almost effective only between nearby nucleons, stronger electromagnetic force will make the β+ decay of each proton combined nearby with other protons stronger and push the dynamic equilibrium between β+ decay of protons and β- decay of neutrons towards the direction to more β+ decay which produce more neutrons; therefore, a stable nucleus would have more neutrons as the number of nuclear charge grows and the maximal number of a stable nucleus can’t exceed 82 if there was no other force took part in.
All these are consistent with the diagram in Figure 1 and are derived from facts we already know. A neutron is not stable by itself, but the number of neutrons can remain constant in a nucleus where they were combined with one or more protons, and the protons combined nearby with each other can have strong electromagnetic force comparable with the nuclear strong force to induce the nearby nucleon to have β+decay, since β+ decay can happen as long as the number of protons in a nucleus exceeds the number when the nucleus with same number of nucleons can be stable, the energy potential a proton could give to the nearby nucleon should be larger than the potential to tear a electron-positron pair from vacuum, all these should be true as they are consistent with the facts and from the reasons we know.
Applying these knowing about the nuclear physics, the fission happens in the unstable nuclides with very large number of nuclear charges, and the remaining products of fission, which would always have more neutrons than protons, would be prone to have β- decay as their N/Z (the ratio between the number of neutrons and protons in a nuclide) would always excess the N/Z by which the nuclides of same element could stably exist. This is true in the remaining nuclear waste of the nuclear reaction cores in Fukushima nuclear power plants, the fission products of Uranium will still be decaying and releasing tremendous energy although the chain reaction had been stopped, and the type of decays is just β- decay. However, these nuclides may be converted to be other stable nuclides by some nuclear reactions that make their N/Z closer to 1:1 or properly larger if Z is larger, and α particle is a good candidate for such operation since it just has a N/Z of 1:1, the nucleus merged with it would have N/Z closer to 1:1, and the initial N/Z is just larger than 1:1. α particle is also safe and stable, since the radioactive nuclides have higher number of nucleons than 100, when the combining energy peaks, the further merge of α particles with the remaining radioactive materials would need absorb but not release energy anymore, more α particle would absorb more energy and possibly more neutrons, the reaction could be safe if the temperature and density of α particles themselves can’t let the fusion therebetween to happen, the merge of α particle with nucleus of heavy element will also absorb a lot of energy. The reaction should be able to proceed since the nuclei must feel like to be stable, and the entropy in a natural process is always conservative. Stable nuclides could form when the original nuclides were merged with supplying α particles, and such merge could absorb the energy which the decaying of the remaining nuclear radioactive material in the reaction cores would release. It is also worth mentioning that the barrier penetration effect must be considered to calculate the condition when nuclear fusion can happen, under temperature of 103K, the probability of an α particle to penetrate the electromagnetic potential barrier between other particles are equal or less than 10(-27), this probability could be less if α is to merge with a nucleus with more protons and this calculation didn’t consider relativity,and the rate that all gas molecules hitting onto the surface of solid material under such temperature can be calculated, process can be safe and happen when the temperature is about 103K and the density of α particles are not high enough,must enable the α particles join into the radioactive nuclei in the remaining reaction cores to form stable nuclei while can’t allow dangerous fusion betweenα particles to happen.1
In conclusion, to provide some thin α particles beams into the reaction cores under proper temperatures about the temperature that the cores will stay when there was no cooling may stop the decaying of the remaining radioactive materials after the chain reaction had been stopped, the fusion of α particles with the nuclei of heavy elements can absorb the remained decaying energy and form stable nuclides which would not be radioactive anymore, and α particles together with its fusion with the nuclei of heavy elements may also absorb the neutrons that may be released in the process and the energy there from to prevent the chain reaction from happening further.
Thanks to everyone.
None.
Author declares that there is no conflict of interest.
- 1. Yáng Fújiā. Atomic Physics. [M]. Higher Education Press, Beijing. Pp.299-314,332-360,369-382.

