Clinical trials face persistent inefficiency, with drug development spanning 10-15 years, device studies requiring 3-7 years, and annual costs exceeding $230 billion globally. Artificial intelligence is fundamentally transforming clinical research through predictive analytics, automation, data integration, and operational optimization. This mini review synthesizes regulatory guidance and empirical evidence from 2020-2025 to examine how AI applications accelerate both drug and device trials and proposes a strategic implementation framework. We conducted systematic searches in PubMed, arXiv, Scopus, and regulatory archives (FDA, EMA) using structured search strings. From approximately 450 initial publications, key sources were selected following rigorous inclusion criteria emphasizing empirical validation and clinical relevance. We developed a four-pillar framework integrating: (1) Predictive Intelligence using machine learning for outcome forecasting; (2) Automation through natural language processing and robotic process automation; (3) Integration of R&D platforms and regulatory systems; and (4) Transparency via explainable AI for regulatory trust.
Evidence demonstrates AI can reduce trial duration by 25-40%, lower operational costs by 30%, and double recruitment speed while improving safety monitoring and patient retention. A case study from Cleveland Clinic demonstrated AI-powered patient identification in approximately 2.5 minutes with 96% accuracy, compared to 427-540 minutes for manual screening with 88- 95% accuracy. When deployed within robust governance structures and internationally coordinated regulatory frameworks, AI integration represents a paradigm shift enabling faster, safer, and more efficient clinical development. Implementation challenges include data standardization barriers, algorithm validation concerns, and the critical need for harmonized global infrastructure to realize AI's transformative potential.
Keywords: Clinical trials, Artificial intelligence, Machine learning, Digital twins, Regulatory automation, Real world evidence
Abbreviations: AI: Artificial Intelligence; CDISC: Clinical Data Interchange Standards Consortium; EMA: European Medicines Agency; FDA: U.S. Food and Drug Administration; GCMLP: Good Clinical and Machine Learning Practice; GCP: Good Clinical Practice; EHR: Electronic Health Record; HIPAA: Health Insurance Portability and Accountability Act; HL7 FHIR: Health Level 7 International Fast Healthcare Interoperability Resources; ICH: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; LLM: Large Language Model; ML: Machine Learning; NLP: Natural Language Processing; RCT: Randomized Controlled Trial; RPA: Robotic Process Automation; RWE: Real- World Evidence; XAI: Explainable AI
Clinical trials serve as the scientific and ethical foundation for validating new drugs and medical devices.1 Despite technological progress, the process remains burdened by inefficiency: approximately 12% of candidate drugs entering Phase I achieve FDA approval with 10-12 years drug development cycle, and device regulatory cycles average 3-7 years.2-4 These persistent delays, driven by fragmented data systems, manual documentation, and limited patient diversity, profoundly impact patient welfare by postponing access to potentially life-saving innovations. The rapid emergence of domain-capable Large Language Models (LLMs) and specialized AI tools since 2022 has created an opportunity for profound change.5,6 Addressing these systemic inefficiencies requires scalable technological interventions capable of managing the complexity inherent in modern clinical research. By integrating computational modeling, Real-World Evidence (RWE) analytics, and genomic profiling, AI enables a holistic approach to trial design and execution.7,8 These technologies are capable of complex, time-intensive tasks - such as protocol generation, precision patient matching, safety signal detection, and regulatory drafting - that traditionally consume extensive expert time.9,10 The challenge is no longer technological viability, but rather the safe, transparent, and scalable integration of AI to maximize patient benefit.11,12 Strategic alignment of trial methodologies with AI capabilities represents a fundamental reimagining of evidence generation, where faster development timelines translate directly into improved patient outcomes and healthcare system efficiency. When trial strategy is optimized through predictive modeling and adaptive designs, the resulting outcomes (shorter durations, cut costs, reduced protocol amendments, and accelerated access to results) translate directly into enhanced patient satisfaction and improved public confidence in medical innovation.13,14
This review examines how strategic alignment of trial methodologies with AI capabilities can fundamentally reimagine evidence generation, potentially accelerating development timelines and improving patient access to innovative therapies.
This mini review synthesizes authoritative literature and regulatory guidance (2020-2025) on artificial intelligence (AI) applications that enhance the efficiency of clinical trials in pharmaceutical and medical device development. The methodology ensured comprehensive yet empirically grounded coverage, emphasizing validated, clinically relevant interventions.
Search strategy: Systematic searches were conducted in PubMed, arXiv, Scopus, and regulatory databases of the FDA and EMA. Boolean search strings combined four domains: (1) AI technologies (machine learning, deep learning, large language models, natural language processing); (2) clinical research processes (clinical trials, drug and device development, recruitment, pharmacovigilance); (3) methodological innovations (adaptive design, digital twins, in-silico modeling, real-world evidence); and (4) regulatory frameworks (FDA and EMA guidance, GCP).
Inclusion and selection criteria: The search identified approximately 450 publications. AI- assisted abstract screening reduced this to 180, followed by human verification and full-text review for methodological rigor and relevance to trial acceleration. Publications were included if they addressed AI in clinical trial design, conduct, monitoring, or regulation; provided empirical evidence or validated frameworks; demonstrated methodological transparency; and appeared in peer-reviewed or regulatory sources. Excluded were unvalidated theoretical works, nonreproducible studies, duplicates, unsupported commentaries, and preclinical studies unrelated to human trials.
Data extraction and synthesis: Extracted data included AI technology type, clinical domain, trial phase, measurable outcomes (efficiency, cost, duration), implementation barriers, regulatory issues, and research gaps. Human researchers synthesized findings, with AI tools used solely for text organization. Quality assessment prioritized methodological transparency, algorithm validation, generalizability, and compliance with FDA AI/ML and ICH E6(R3) Good Clinical Practice standards.
Framework development: Based on the synthesized evidence, we developed a strategic four- pillar framework for AI integration in clinical trials: Predictive Intelligence, Automation, Integration, and Transparency. This framework was constructed through iterative analysis of successful implementation patterns, regulatory requirements, and identified gaps in existing industry models. This framework introduces ethical governance and data interoperability as foundational elements and ensures that the review findings accurately represent the current state of AI applications in clinical research while providing actionable guidance for researchers, regulators, and industry stakeholders seeking to modernize clinical development processes.
To modernize clinical trials for both drug and device programs, we propose a strategic framework built on four pillars: Predictive Intelligence, Automation, Integration, and Transparency. This framework diverges from prior industry models such as Deloitte's Intelligent Trials and the FDA AI/ML Action Plan,3,13 which primarily focus is on automation of discrete processes or compliance-oriented guidance Figure 1.
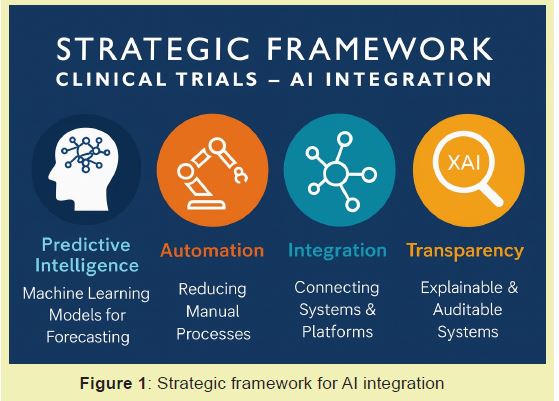
Predictive intelligence: Utilizing machine learning (ML) models trained on historical data, RWE, and molecular profiles to forecast clinical outcomes, safety risks, and patient responses.7,15 The use of federated learning allows data to remain secure in local databases while training centralized models, enabling multi-site collaboration without compromising patient privacy.10,16
Automation: Employing Natural Language Processing (NLP), Robotic Process Automation (RPA), and intelligent workflow orchestration to reduce manual data handling, documentation cycles, and repetitive analytical tasks.6
Integration: Connecting R&D platforms, regulatory information systems, and electronic data capture through interoperable AI-enabled architectures to eliminate data silos.15
Transparency: Maintaining explainable, auditable AI systems (Explainable AI - XAI) with clear documentation of model training, validation, and decision pathways to establish regulatory and patient trust.12,17
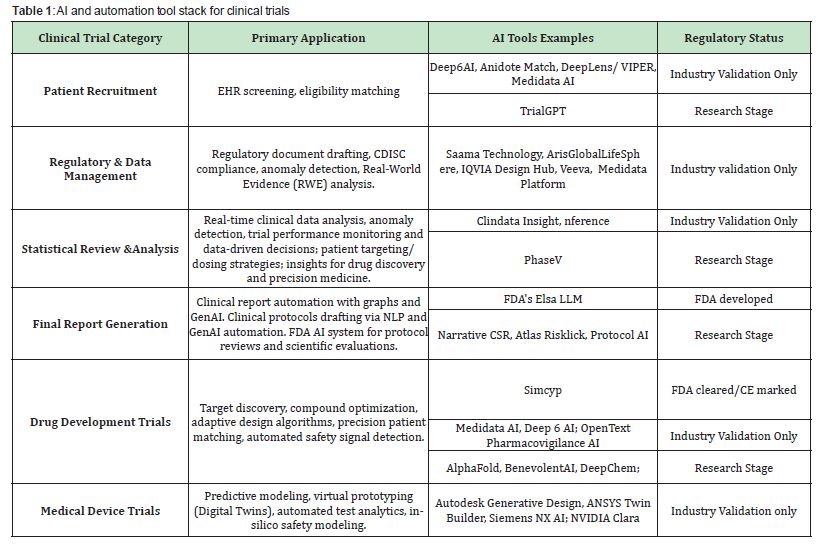
Table 1 offers examples of existing AI and Automation tool stack for clinical studies within the discussed framework.
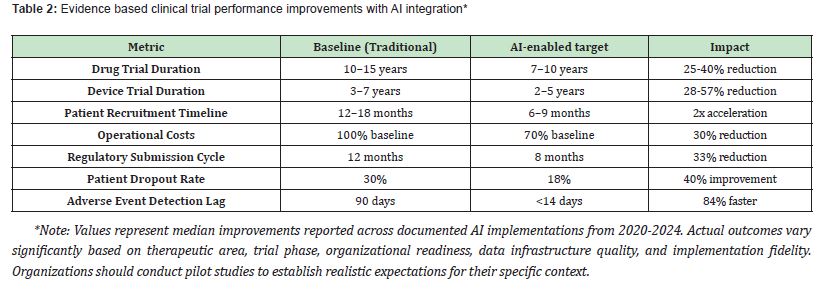
Overview of AI implementation landscape: The current clinical trials landscape, marked by delays in recruitment (accounting for 30% of tri al timelines) and high data cleaning costs.2,13 AI interventions address these specific inefficiencies through predictive patient identification, automated quality control, and intelligent protocol adaptation.8,9 AI interventions generate substantial and quantifiable efficiencies across the development lifecycle, as detailed in Table 2.11,13
Precision patient selection and retention: AI algorithms for patient screening demonstrate accuracy ranging from 72-85% in identifying eligible candidates through EHR analysis, genomic databases, and RWE integration, compared to 60-68% accuracy with manual screening.8,15 Performance varies by inclusion criteria complexity and data quality.
Digital twins and in-silico modeling: For medical devices (as an example), digital twin technology - which creates virtual models of the patient or the device/organ interaction - enables rigorous in-silico testing before human trials.18,19 Simulations, such as computational fluid dynamics for cardiovascular devices, reduce the number of required human subjects while maintaining statistical power and regulatory confidence.20 The FDA's ENRICHMENT project and collaboration with industry partners demonstrates institutional acceptance of these virtual twin methodologies.21-23 Digital Twin technology is also used for drug development by creating virtual models of biological systems (from cells to patients) to simulate and test drugs in a digital environment.
Adaptive trial design and safety monitoring: ML algorithms enable Bayesian adaptive designs that continuously monitor interim data to optimize dosing, sample size, and endpoint selection in real-time.24 This can reduce trial duration while maintaining strict Type I error control. In safety monitoring, AI pharmacovigilance systems detect adverse event patterns significantly faster (14 days vs. 90 days), analyzing RWE streams and patient-reported outcomes to stratify patient risk profiles.8,25
Case study: Cleveland Clinic piloted Dyania Health's Synapsis AI platform across oncology, cardiology, and neurology to improve patient identification for clinical trials.26 In a melanoma trial, Synapsis AI identified eligible patients in about 2.5 minutes with 96% accuracy, compared to 427 minutes for a specialized nurse (95% accuracy) and 540 minutes for an oncology research nurse (88% accuracy). This represents a major reduction in screening time while maintaining or improving accuracy. However, the study was a pilot and reflects potential eligibility only.
Broader applicability to other trial types or sites with less structured data remains uncertain.
This section explores the challenges and barriers associated with five key AI efficiency drivers across the trial lifecycle: high-quality data, algorithms, ethics, change management, and infrastructure.
Data quality and standardization barriers: AI algorithms are dependent on high-quality, standardized input data. Clinical data remains fragmented across incompatible systems with inconsistent terminologies and missing values. Investment in harmonization standards like CDISC and HL7 FHIR remains essential.15,27
Algorithm validation and generalizability concerns: Most AI models are trained on datasets from high-income regions, creating risks of algorithmic bias and poor generalization. Continuous revalidation is crucial.28,29
Ethical and legal challenges: Emerging ethical issues include accountability for AI-driven clinical decisions, data provenance, and the reproducibility of adaptive algorithms.30 Recent publications underscore the need for transparency in human-AI collaboration, emphasizing auditability and explainability as prerequisites for regulatory and ethical trust.11,12,14
Change management: While evidence demonstrates significant potential benefits, successful AI integration requires substantial upfront investment in data infrastructure, staff training, and change management. Organizations should anticipate 12–24-month implementation timelines for comprehensive AI adoption, with incremental deployment recommended for risk mitigation and stakeholder buy-in. While AI offers tremendous promise, several substantive challenges must be acknowledged to ensure realistic expectations and responsible deployment.14,30
The integration of artificial intelligence into clinical trials faces several persistent concerns, each with evolving solutions and ongoing challenges. Regarding regulatory acceptance, the FDA's Good Machine Learning Practice defines validation standards,31 ICH E6(R3) includes guidance on AI and computerized systems,1 the EMA's AI Task Force provides direction on AI in medicine,32 and over 900 AI-enabled medical devices have been approved by the FDA.33 However, these evolving frameworks demand adaptive validation approaches with early regulator engagement, while post-market monitoring requirements continue to tighten and regional regulatory differences across FDA, EMA, and PMDA persist. Concerns about AI replacing clinicians are being addressed through a fundamental shift in perspective: AI augments rather than replaces clinicians, with clinicians retaining ethical and decision-making authority34 while AI manages routine data tasks to enable more complex, patient-centered care. "Human-in-the-loop" models35 remain the standard, though successful AI integration will require enhanced clinician training in AI literacy and interpretability, along with evolving liability frameworks and collaboration models tailored to specific tasks and risk levels.
The black box" opacity problem is being tackled through multiple transparency initiatives. Explainable AI36 improves transparency, regulatory systems maintain audit trails and version control, CONSORT-AI and SPIRIT-AI ensure transparent clinical reporting, and model cards with datasheets standardize documentation. Looking ahead, transparency requirements must scale appropriately with risk levels, stakeholders must navigate trade-offs between model performance and explainability, and continuous monitoring systems are needed to address model drift over time.
Finally, data privacy concerns37 are being managed through technical innovations: federated learning keeps data decentralized, HIPAA-compliant encryption and de-identification protect sensitive information, differential privacy adds mathematical privacy guarantees, and synthetic data supports model training without exposing real patient information. Future considerations include harmonizing privacy governance across global regulatory frameworks like GDPR, HIPAA, and PIPL, addressing potential quantum computing threats to current encryption methods, and maintaining the delicate balance between privacy protection and data utility for effective model development.
Global infrastructure imperative: Clinical trials have evolved dramatically since their formalization in the mid-20th century, with the 1947 Nuremberg Code establishing ethical principles and the 1962 Kefauver-Harris Amendment mandating efficacy demonstration. Today's landscape reflects profound globalization: approximately 40-50% of FDA-regulated clinical trial sites operate outside the United States, with enrollment extending across Asia, Latin America, Eastern Europe, and Africa to access diverse patient populations, reduce costs, and accelerate recruitment timelines. This internationalization brings substantial benefits – enhanced generalizability, increased enrollment rates, and cost efficiencies - but also introduces formidable challenges including data quality heterogeneity, regulatory compliance across jurisdictions, and infrastructure disparities that directly impact data integrity and patient safety.38,39
As we advance toward AI-enabled clinical trials, a critical reality must be acknowledged: Artificial intelligence, regardless of its sophistication, cannot resolve fundamental problems of data operability and quality if the underlying international regulatory frameworks and technological infrastructure remain fragmented. AI algorithms trained on inconsistent data standards, incompatible electronic systems, and divergent regulatory requirements will perpetuate rather than eliminate existing inefficiencies. The promise of AI-driven acceleration in Section 4 is contingent upon establishing harmonized global standards for data exchange (such as universal adoption of CDISC and HL7 FHIR),40 mutual recognition agreements for AI validation across regulatory authorities, and equitable technology infrastructure that enables real- time data integration from diverse geographic sites. Without coordinated international efforts to standardize protocols, ensure interoperable systems, and establish unified AI governance frameworks, we risk creating a technologically advanced yet structurally siloed ecosystem where AI tools optimize individual components while the broader system remains inefficient. The FDA's International Council for Harmonization (ICH) guidelines and EMA's multinational initiatives represent essential steps, but comprehensive global coordination - extending beyond high-income nations to include emerging research hubs - remains imperative for realizing AI's transformative potential in truly global clinical development programs.1
Future directions and conclusion
AI integration transforms clinical trials from linear, siloed processes into dynamic, adaptive systems that accelerate discovery and improve patient outcomes.9,15 For sustained progress, the sector must address key policy and regulatory gaps.3,4 We recommend establishing global standards for AI auditing and validation, aligned with frameworks like Good Clinical Machine Learning Practice (GCMLP).1 This initiative should integrate directly with ongoing regulatory efforts, including the FDA's Artificial Intelligence and Machine Learning Software as a Medical Device (AI/ML SaMD) framework,3,31,32 which provides a reference structure for continuous learning systems. Regulatory bodies should also expedite the development of AI- specific pathways for in-silico evidence and mandate transparent interoperability standards.21,22
AI integration in clinical trials has progressed from theoretical promise to demonstrated reality, with documented improvements in efficiency, safety monitoring, and patient outcomes. As regulatory frameworks mature and standardization efforts advance, AI will transition from optional enhancement to essential infrastructure.5,9 This evolution promises to reshape biomedical innovation, enabling more equitable access to safe, effective therapeutics while reducing development timelines and costs. However, realizing this potential requires continued collaboration among researchers, regulators, industry, and ethicists to ensure AI systems remain transparent, validated, and aligned with patient welfare as the primary objective.
I.I. Agoulnik conceived the review framework, conducted literature search and analysis, drafted the manuscript, and approved the final version.
This is a mini review article based on publicly available published literature. No original datasets were generated or analyzed during this study.
Not applicable for review article.
AI-assisted tools were used for literature abstract screening and sentence-level clarity improvement in selected sections. All scientific content, analysis, synthesis, and conclusions represent the author's original intellectual contribution. No AI-generated text was used without human review and modification. All content and conclusions represent the author’s original contribution.
The author expresses gratitude to Stephy Publishers for the invitation to contribute to Current Investigations in Clinical and Medical Research. Special thanks to AIiIA Consulting for strategic vision and manuscript writing.
No external funding was received for this work.
The author declares no conflicts of interest. The author is affiliated with AIiIA Consulting, which provides AI strategy consulting services. This affiliation did not influence the content or conclusions of this review.
- 1. International Council for Harmonization. ICH E6(R3) Guideline on Good Clinical Practice. 2023.
- 2. McKinsey & Company. The bio revolution: Innovations transforming economies, societies, and our lives. 2023.
- 3. U.S. Food and Drug Administration. Artificial Intelligence/Machine Learning: The New Frontier of Drug Development. FDA Guidance. 2024.
- 4. Congressional Budget Office. Research and Development in the Pharmaceutical Industry. 2021.
- 5. Rajpurkar P, Chen E, Banerjee O, et al. AI in health and medicine. Nat Med. 2022;28(1):31-38.
- 6. Hogan Lovells. FDA's Evolving Regulatory Framework for AI Use in Drug & Device Clinical Trials and Research. 2025.
- 7. Obaido G, Mienye ID, Egbelowo OF, et al. Supervised machine learning in drug discovery and development: Algorithms, applications, challenges, and prospects. Mach Learn Appl. 2024;17:100576.
- 8. Anuyah S, Singh MK, Nyavor H. Advancing clinical trial outcomes using deep learning and predictive modelling: bridging precision medicine and patient-centered care. arXiv. 2024.
- 9. Linghua Yu. Medical Artificial Intelligence and Human Values. N Engl J Med. 2024;391(12):1166-1168.
- 10. Aidoc. Deep learning in healthcare: The ultimate guide. 2025.
- 11. Chen Y, Ohlssen D, Readie A, et al. The Framework That Survives Bad Models: Human-AI Collaboration For Clinical Trials. arXiv. 2025.
- 12. Bajwa J, Munir U, Nori A, et al. Artificial intelligence in healthcare: Transforming the practice of medicine. Future Healthc J. 2021;8(2):e188-e194.
- 13. Deloitte Insights. Intelligent clinical trials: Unlocking the power of AI in pharma and life sciences. 2024.
- 14. Lee C, Lee A. How Artificial Intelligence Can Transform Randomized Controlled Trials. Transl Vis Sci Technol. 2020;9(2):9.
- 15. Kaissis GA, Makowski MR, Rückert D, et al. Secure, privacy-preserving and federated machine learning in medical imaging. Nat Mach Intell. 2020;2:305-311.
- 16. Rieke N, Hancox J, Li W, et al. The future of digital health with federated learning. NPJ Digit Med. 2020;3:119.
- 17. Amann J, Blasimme A, Vayena E, et al. Explainability for Artificial Intelligence in Healthcare: A Multidisciplinary Perspective. BMC Med Inform Decis Mak. 2020;20(1):310.
- 18. Tudu C, Sharma S, Kumar D. Computational Modeling and Digital Twin Technologies in Medical Device Development. Biomed Mater Devices. 2025.
- 19. Applied Clinical Trials Online. A New Regulatory Road in Clinical Trials: Digital Twins. 2025.
- 20. Venkatesh KP, Raza MM, Kvedar JC. Health digital twins as tools for precision medicine: Considerations for computation, implementation, and regulation. NPJ Digit Med. 2022;5(1):150.
- 21. Applied Clinical Trials Online. Understanding FDA and EMA Guidance on AI and Digital Twin Applications. 2025.
- 22. Tasmurzayev N, Amangeldy B, Imanbek B, et al. Digital Cardiovascular Twins, AI Agents, and Sensor Data: A Narrative Review from System Architecture to Proactive Heart Health. Sensors. 2025;25(17):5272.
- 23. Coorey G, Figtree GA, Fletcher DF, et al. The health digital twin to tackle cardiovascular disease-A review of an emerging interdisciplinary field. NPJ Digit Med. 2022;5:126.
- 24. Poondla N, Brathwaite JS, Sheth M. Unlocking the power of digital biomarkers in clinical trials. Assoc Clin Res Prof (ACRP). 2025.
- 25. The Guardian. AI could make it harder to establish blame for medical failings. 2025.
- 26. Cleveland Clinic Newsroom. Cleveland Clinic Accelerates Clinical Trial Recruitment with Roll Out of Dyania Health's Artificial Intelligence Platform Across Health System. 2025.
- 27. Baker RL, Hamidi M, McKenzie L, et al. The Story Behind the HL7 FHIR to CDISC Mapping Implementation Guide. J Soc Clin Data Manag. 2024;4(1).
- 28. Wismüller A, Stockmaster L. A Prospective Randomized Clinical Trial for Measuring Radiology Study Reporting Time on Artificial Intelligence-Based Detection of Intracranial Hemorrhage in Emergent Care Head CT. arXiv. 2020.
- 29. Plana D, Shung DL, Grimshaw AA, et al. Randomized Clinical Trials of Machine Learning Interventions in Health Care: A Systematic Review. JAMA Netw Open. 2022;5(9):e2233946.
- 30. Mateen BA, Moorthy V, Labrique A, et al. Artificial intelligence and clinical trials: a framework for effective adoption. Lancet Dig Health. 2025;7(8):100898.
- 31. U.S. Food and Drug Administration (FDA). Good Machine Learning Practice for Medical Device Development: Guiding Principles. 2021.
- 32. European Medicines Agency (EMA). Science Medicine Health: Artificial Intelligence. 2025.
- 33. MedTech Dive. AI in medtech is booming. 2025.
- 34. Federation of State Medical Boards (FSMB). Navigating the Responsible and Ethical Incorporation of Artificial Intelligence into Clinical Practice. 2024.
- 35. Notable. Why Healthcare Keeps a Human-in-the-Loop. 2025.
- 36. Sadeghi Z, Alizadehsani R, CIFCI MA, et al. A Review of Explainable Artificial Intelligence in Healthcare. Comput Biol Med. 2024;118(Pt A):109370.
- 37. Dyda A, Purcell M, Curtis S, et al. Differential Privacy for Public Health Data: An Innovative Tool to Optimize Information Sharing While Protecting Data Confidentiality. Patterns (N Y). 2021;2(12):100366.
- 38. Morgan Lewis. Key Considerations for Foreign Clinical Trials When Looking Abroad for Product Development. 2025.
- 39. Axios. FDA launches agencywide AI tool. 2025.
- 40. Markus AF, Kors JA, Rijnbeek PR. The role of explainability in creating trustworthy artificial intelligence for health care. J Biomed Inform. 2021;113:103655.

