Schaaf-Yang syndrome (SYS) is an imprinted neurodevelopmental disorder caused by truncating variants in the paternally expressed MAGEL2 gene within chromosome 15q11-q13. Early clinical features overlap with Prader-Willi syndrome (PWS), yet SYS is characterized by distal joint contractures and a higher prevalence of autistic traits. Recent molecular and animal studies have advanced understanding of MAGEL2 function and identified potential therapeutic targets. Molecular confirmation through MAGEL2 sequencing remains the diagnostic gold standard. Current treatments are primarily supportive; early oxytocin administration has demonstrated partial behavioral improvements in animal models, likely via modulation of hypothalamic circuits, though human evidence is lacking. Comprehensive natural history data and genotype-phenotype correlations are still limited. Effective management requires a coordinated, multidisciplinary approach, and further translational studies are essential to guide targeted therapies.
Keywords: Schaaf-Yang syndrome, MAGEL2 gene, Neurodevelopmental disorder, Prader-Willi syndrome, Oxytocin
Schaaf-Yang syndrome (SYS) is an ultra-rare genetic disorder first described by Dr. Christian Patrick Schaaf in 2013. The condition is caused by truncating mutations in the paternally inherited MAGEL2 gene. MAGEL2 belongs to the melanoma antigen (MAGE) family and is located within the Prader-Willi critical region on chromosome 15q11-q13.1-3 These nonsense or frameshift variants lead to the production of a truncated protein, disrupting normal MAGEL2 function and resulting in the characteristic clinical manifestations of the syndrome.4 Both Schaaf-Yang syndrome (SYS) and Prader-Willi syndrome (PWS) share several clinical characteristics, including neonatal hypotonia, feeding difficulties during infancy, and global developmental delay. However, the two conditions can be distinguished by certain key features, with autism spectrum disorder and joint contractures being more prominent in SYS.1
In this study, we aim to provide a comprehensive evaluation of the clinical and genetic characteristics of Schaaf-Yang syndrome to enhance understanding of its phenotypic spectrum and underlying molecular mechanisms, addressing the current gaps in clinical recognition and regional epidemiological data.
Schaaf-Yang syndrome (SYS) presents with a broad yet distinctive clinical profile primarily involving the nervous and musculoskeletal systems. Early-life hypotonia and feeding difficulties often resemble Prader-Willi syndrome (PWS), but later features such as joint contractures and autism spectrum behaviors help distinguish SYS as a separate entity. The variability in symptom severity reflects the complex effects of MAGEL2 dysfunction on development.
Neonatal and early life
During the neonatal period, patients with Schaaf-Yang syndrome (SYS) commonly exhibit marked hypotonia and weak sucking reflexes, often necessitating assisted or specialized feeding methods. These early-life manifestations resemble those observed in Prader-Willi syndrome (PWS), reflecting the shared disruption of genes within the 15q11-q13 region. However, certain distinguishing features, such as congenital joint contractures—particularly involving the small finger joints—and the absence or delayed onset of hyperphagia, help differentiate SYS from the classical PWS phenotype.5 Beyond hypotonia and feeding difficulties, affected infants frequently experience poor weight gain and failure to thrive, requiring prolonged nutritional support.1,4 Respiratory challenges, including transient apnea and increased susceptibility to infections, are also commonly reported during the first weeks of life, reflecting the systemic impact of early muscular hypotonia.4 Moreover, subtle prenatal signs such as reduced fetal movements or decreased intrauterine activity have been observed in several cases, suggesting that abnormal neuromuscular development may precede birth.1 Collectively, these neonatal and early-life features define the initial clinical signature of SYS and underscore the importance of early recognition for timely intervention and supportive care Figure 1.
Growth and endocrine
Patients with Schaaf-Yang syndrome frequently exhibit endocrine abnormalities, with growth hormone deficiency being the most commonly reported. This deficiency often manifests as hypoglycemia in early childhood, sometimes leading to seizures or failure to thrive. Additional hormonal disturbances, such as central hypothyroidism, may also occur, reflecting a broader pituitary dysfunction in a subset of individuals. While some endocrine deficits improve over time, early recognition and management are crucial to prevent metabolic complications and support normal growth and development.6 In Schaaf-Yang syndrome, endocrine assessment often reveals growth hormone deficiency, which is confirmed through stimulation tests such as the GHRH-arginine and clonidine tests, with a peak GH level below 20mU/L considered diagnostic. Pubertal abnormalities may also occur, including delayed or precocious onset, and should be evaluated using standard clinical criteria. In addition, some patients exhibit central adrenal insufficiency or thyroid dysfunction, underscoring the importance of comprehensive hormonal evaluation and early management to support growth and metabolic stability.8
Neurodevelopmental and behavioral
Patients with Schaaf-Yang syndrome commonly exhibit global neurodevelopmental delay, hypotonia, and early-life autonomic disturbances. Characteristic EEG patterns observed in some individuals may reflect underlying hypothalamic dysfunction, correlating with both motor and behavioral impairments. While certain neurodevelopmental deficits may improve over time, persistent challenges in motor coordination, adaptive responses, and feeding are frequently reported.11 Even among patients sharing the same MAGEL2 mutation, neurodevelopmental and behavioral features can vary widely. Common manifestations include deficits in social communication and behaviors consistent with autism spectrum disorder. This clinical heterogeneity underscores the importance of individualized assessment and early supportive interventions to optimize developmental outcomes in affected individuals.14
Dysmorphic and musculoskeletal
Patients with Schaaf-Yang syndrome frequently exhibit distinctive musculoskeletal and dysmorphic traits. Neonatal hypotonia is a near-universal early feature, often accompanied by feeding difficulties. Joint contractures, ranging from mild interphalangeal involvement to severe arthrogryposis, are commonly observed, along with various hand anomalies such as tapering fingers, clinodactyly, camptodactyly, brachydactyly, and adducted thumbs. Skeletal assessments often reveal small hands and feet, short stature, and spinal deformities including scoliosis or kyphosis. Dysmorphic features extend to the eyes, with strabismus, esotropia, and myopia reported, as well as early manifestations of hypogonadism in males and delayed sexual elopment in females. Sleep disturbances, particularly sleep apnea, are also prevalent in affected individuals, reflecting the systemic impact of these musculoskeletal and dysmorphic abnormalities.5 Severe musculoskeletal abnormalities in Schaaf-Yang syndrome, including pronounced joint contractures and spinal deformities such as scoliosis, contribute significantly to long-term morbidity. These structural anomalies can exacerbate hypotonia-related functional impairments, impact mobility, and increase susceptibility to secondary complications such as sleep disturbances or respiratory challenges. Early recognition and appropriate management of these musculoskeletal features are crucial to improve quality of life and reduce potential long-term health risks.10
Systemic and additional
Beyond early-life complications, SYS can affect multiple organ systems throughout development. Sleep disturbances, including obstructive sleep apnea, are prevalent, and gastrointestinal dysmotility may contribute to chronic feeding difficulties. Additionally, some patients exhibit metabolic or endocrine abnormalities that interact with systemic health, highlighting the importance of longitudinal monitoring to anticipate and manage multisystem involvement.5 Long-term morbidity in Schaaf-Yang syndrome often arises from cumulative effects of musculoskeletal, respiratory, and endocrine complications. Severe joint contractures, spinal deformities, and persistent hypotonia can exacerbate functional limitations, while systemic issues such as recurrent infections and respiratory insufficiency may contribute to increased mortality risk. These findings underscore the need for ongoing, coordinated care to address the multisystemic impact of the syndrome and improve long-term outcomes.10
Comparison with PWS
Schaaf-Yang syndrome shares several clinical features with Prader-Willi syndrome, including neonatal hypotonia, feeding difficulties, and global developmental delay. However, truncating MAGEL2 mutations in SYS result in distinctive phenotypes that help differentiate it from classical PWS. Autism spectrum disorder and congenital joint contractures are markedly more prevalent in SYS, whereas features typical of PWS such as pronounced hyperphagia and subsequent obesity are often absent or only mildly expressed. Genetic analyses further underscore these differences, as SYS arises specifically from pathogenic variants in the paternally expressed MAGEL2 gene, while PWS involves larger deletions or disruptions of multiple genes within the 15q11-q13 region. Collectively, these observations highlight both the phenotypic overlap and the distinguishing features that are critical for accurate diagnosis and management.9 While Schaaf–Yang syndrome (SYS) and Prader-Willi syndrome (PWS) share a common genetic locus within the 15q11-q13 region, the pathogenic mechanisms underlying these disorders differ substantially. SYS results from truncating mutations in the paternally expressed MAGEL2 gene, whereas PWS arises from larger deletions or maternal uniparental disomy affecting multiple imprinted genes in the same chromosomal region. This distinction explains the overlap in clinical features, such as hypotonia and developmental delay, alongside key differentiating traits, including joint contractures and autism spectrum behaviors that are characteristic of SYS. The comparative molecular analyses of these syndromes provide deeper insights into the role of individual imprinted genes, particularly MAGEL2, in hypothalamic and neurobehavioral regulation.13
In summary, the clinical phenotype of SYS is diverse but characterized by consistent neurodevelopmental and behavioral features. Understanding its genetic basis is crucial for clarifying this variability and improving diagnostic accuracy. The next section therefore focuses on the molecular mechanisms underlying MAGEL2-related pathology.
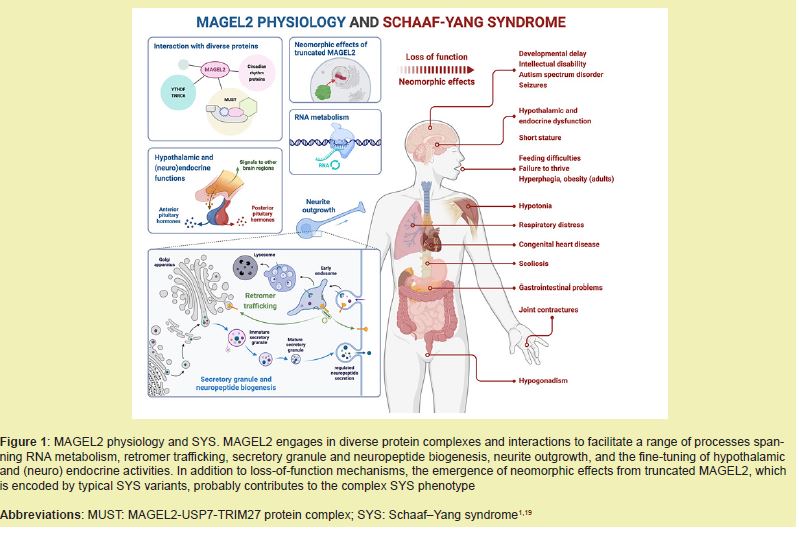
Schaaf-Yang syndrome arises from truncating mutations in the paternally expressed MAGEL2 gene within the 15q11-q13 region. These mutations lead to a loss of normal MAGEL2 function, which plays a crucial role in hypothalamic development, protein ubiquitination, and neuronal maturation. Disruption of these processes contributes to the multisystem phenotype observed in SYS, including neurodevelopmental delay, endocrine abnormalities, and musculoskeletal anomalies.17
Research focused on the cellular level, notably by Castilla-Vallmanya,1 demonstrates that MAGEL2 dysfunction hinders endosomal recycling and ubiquitin-mediated protein degradation. This impairment is strongly implicated as a mechanism for the abnormal neuronal connectivity and altered neurotransmission observed in SYS patients. Furthermore, investigations into the protein's function suggest that the N-terminal domain of MAGEL2 is integral to RNA metabolism and post-transcriptional control. It is hypothesized that mutations within this domain disrupt crucial processes like mRNA handling and protein translation, potentially explaining the early-onset symptoms such as hypotonia, feeding difficulties, and cognitive deficits.3,16
Truncating mutations in MAGEL2 generate phenotypes that partially overlap with Prader-Willi syndrome but are distinguished by joint contractures and autism spectrum traits. Studies indicate that specific MAGEL2 variants contribute directly to hypothalamic dysfunction, affecting thermoregulation, appetite regulation, and endocrine homeostasis. Beyond intracellular mechanisms, MAGEL2 dysfunction impacts systemic physiology, particularly through oxytocin-mediated pathways, which may impair social behaviors, stress responses, and thermoregulation. These genotype-phenotype correlations emphasize the importance of precise molecular characterization in predicting clinical outcomes.2,7
Schaaf-Yang syndrome (SYS) is an ultra-rare neurodevelopmental disorder with an estimated prevalence of less than 1 in 50,000 individuals worldwide. Over 250 patients with paternally inherited pathogenic variants in the MAGEL2 gene have been identified to date, although only approximately 120 cases had been reported in the literature as of September 2020.5,10 The rarity of SYS is further reflected in its variable phenotypic presentation, which includes neurodevelopmental delay, hypotonia, feeding difficulties, and musculoskeletal anomalies. Morbidity and mortality are influenced by the degree of multisystem involvement, particularly in the neonatal period, highlighting the importance of early diagnosis and clinical monitoring.10 Despite global case reports, certain regions remain underrepresented. This lack of regional reporting underscores the need for increased clinical awareness, systematic genetic testing, and epidemiological studies to accurately determine prevalence, identify novel MAGEL2 variants, and guide patient management strategies.4
Although Schaaf-Yang syndrome (SYS) remains classified as an ultra-rare disorder, recent multicenter analyses have revealed an increasing number of genetically confirmed cases, suggesting that the prevalence may be underestimated due to limited clinical recognition and diagnostic accessibility. Data derived from longitudinal cohort studies indicate that many patients are initially misdiagnosed with Prader-Willi syndrome or other neurodevelopmental disorders, delaying molecular confirmation and epidemiological reporting. Furthermore, improvements in next-generation sequencing technologies and the expansion of genetic testing panels have contributed to a gradual rise in the identification of MAGEL2 variants in recent years. These findings highlight the evolving nature of SYS epidemiology and the importance of global registries for accurate case documentation and genotype-phenotype correlation.15
The diagnosis of Schaaf-Yang syndrome (SYS) relies primarily on molecular confirmation of truncating variants in the paternally expressed MAGEL2 gene. Prenatal and early postnatal recognition remains challenging due to the nonspecific nature of neonatal hypotonia, feeding difficulties, and subtle dysmorphic features. Prenatal ultrasound may occasionally reveal reduced fetal movements, but these findings are neither sensitive nor specific. Comprehensive genetic testing, including whole-exome sequencing or targeted MAGEL2 analysis, is essential for accurate diagnosis and early clinical management.12
Phenotypic variability among SYS patients can complicate differentiation from overlapping syndromes such as Prader-Willi syndrome (PWS) and Chitayat-Hall syndrome. Even individuals sharing identical MAGEL2 mutations may exhibit distinct clinical features, including differences in joint contractures, developmental delay severity, and autism spectrum behaviors. Therefore, integrating detailed clinical assessment with molecular testing is crucial to distinguish SYS from related neurodevelopmental disorders and to guide appropriate intervention strategies.14
Management of Schaaf-Yang syndrome (SYS) requires a multidisciplinary and symptom-oriented approach, reflecting the syndrome’s wide-ranging neurological, endocrine, and musculoskeletal manifestations. Early diagnosis is critical to initiate timely interventions and to prevent secondary complications, particularly during infancy when hypotonia and feeding difficulties are most pronounced. Comprehensive care often involves coordination between neurologists, endocrinologists, geneticists, and rehabilitation specialists. Regular monitoring of growth parameters, nutritional status, and neurodevelopmental milestones remains essential for optimizing long-term outcomes and improving overall quality of life.4,10
Endocrine dysfunctions are among the most treatable aspects of SYS, and their management can substantially enhance developmental progression. Growth hormone replacement therapy has shown favorable outcomes in selected patients with growth hormone deficiency, improving muscle tone, linear growth, and metabolic regulation. Likewise, evaluation and treatment of thyroid or adrenal insufficiencies should be guided by standard hormonal assays and clinical findings. In patients with delayed or abnormal pubertal development, hormone replacement may be indicated to promote appropriate sexual maturation and bone health. Systematic endocrine follow-up is therefore an integral part of clinical management in SYS.6
Beyond endocrine therapy, rehabilitative and behavioral interventions form the cornerstone of long-term management. Early implementation of physiotherapy and occupational therapy aids in improving joint mobility and reducing the impact of congenital contractures, while speech and feeding therapy can address dysphagia and communication difficulties. Behavioral therapy and structured educational support are essential for managing autistic traits and social communication deficits, which are prevalent in SYS. Family counseling and social support services are equally important to address the psychosocial aspects of living with a chronic rare disorder, underscoring the value of sustained, individualized, and multidisciplinary care Figure 2.5,10
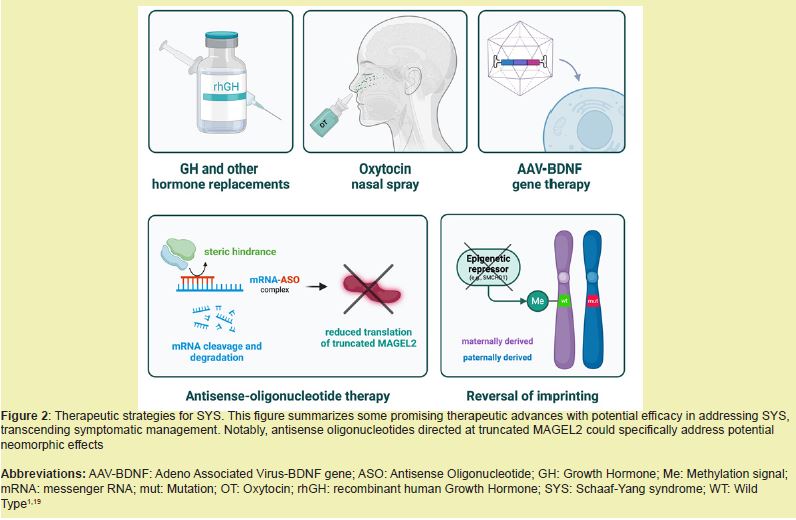
Despite significant progress in understanding Schaaf-Yang syndrome (SYS), numerous gaps remain in elucidating its full molecular, neurodevelopmental, and clinical spectrum. Future research should focus on comprehensive genotype-phenotype correlations to predict individual patient outcomes and identify potential biomarkers for early diagnosis. Longitudinal studies are needed to characterize developmental trajectories and multisystem involvement, which could guide personalized therapeutic interventions. Additionally, exploration of novel molecular pathways, including synaptic regulation and neuroendocrine signaling, may reveal targeted strategies to mitigate behavioral, cognitive, and endocrine manifestations. Collaborative, multicenter studies and shared patient registries will be essential to expand sample sizes, validate findings, and accelerate translation from molecular insights to effective clinical management.
This comprehensive review affirms that Schaaf-Yang syndrome (SYS) is a distinct neurodevelopmental disorder, predominantly caused by truncating mutations in the paternally expressed MAGEL2 gene. Its unique phenotype, marked by neonatal hypotonia, joint contractures, global developmental delay, and a high prevalence of Autism Spectrum Disorder (ASD), is crucial for differentiating it from the phenotypically overlapping Prader-Willi Syndrome (PWS). Moving forward, research must pivot from purely supportive care toward targeted molecular therapies. The discovery that the truncated MAGEL2 protein is stable and localizes to the nucleus suggests a pathogenic neomorphic effect. This insight, combined with the need to explore biomarkers like OXTR methylation to personalize oxytocin-based behavioral therapies, defines the cutting edge of translational research for SYS, aiming to elucidate the full pathophysiology and guide precision medicine strategies.
None.
This Review Article received no external funding.
Regarding the publication of this article, the authors declare that they have no conflicts of interest.
- 1. Castilla Vallmanya L, Centeno Pla M, Serrano M, et al. Advancing in Schaaf-Yang syndrome pathophysiology: from bedside to subcellular analyses of truncated MAGEL2. J Med Genet. 2023;60(4):406-415.
- 2. Asadi S. Pathology in Medical Genetic Books (26 Vol), Amidi Publications, Iran 2017-2025.
- 3. Dos Santos HM, Valente LMV, Dos Santos RWV, et al. The Diagnostic and Therapeutic Challenges of Schaaf-Yang Syndrome: A Brazilian Case Report. J Child Neurol. 2025;8830738251360215.
- 4. Fountain MD, Aten E, Cho MT, et al. The phenotypic spectrum of Schaaf-Yang syndrome: 18 new affected individuals from 14 families. Genet Med. 2017;19(1):45-52.
- 5. Heimdörfer D, Vorleuter A, Eschlböck A, et al. Truncated variants of MAGEL2 are involved in the etiologies of the Schaaf-Yang and Prader-Willi syndromes. Am J Hum Genet. 2024;111(7):1383-1404.
- 6. Maaß JG, Brennenstuhl H, Schaaf CP. Morbidity and mortality in Schaaf-Yang syndrome. Ann Transl Med. 2023;11(12):405.
- 7. Pan L, Zheng L, Wu X, et al. A short period of early life oxytocin treatment rescues social behavior dysfunction via suppression of hippocampal hyperactivity in male mice. Mol Psychiatry. 22;27:4157-4171.
- 8. Juriaans AF, Kerkhof GF, Hokken Koelega ACS. The spectrum of the Prader-Willi-like pheno- and genotype: A review of the literature. Endocr Rev. 2022;43(1):1-18.
- 9. Juriaans AF, Kerkhof GF, Garrelfs M, et al. Schaaf-Yang Syndrome: Clinical phenotype and effects of 4 years of growth hormone treatment. Horm Res Paediatr. 2024;97(2):148-156.
- 10. Nunes S, Xavier M, Lourenço C, et al. Schaaf-Yang Syndrome: A real challenge for prenatal diagnosis. Cureus. 2021;13(12):e20414.
- 11. Sanderson MR, Fahlman RP, Wevrick R. The N-terminal domain of the Schaaf-Yang syndrome protein MAGEL2 likely has a role in RNA metabolism. J Biol Chem. 2021;297(2):100959.
- 12. Camerino C. The pivotal role of oxytocin's mechanism of thermoregulation in Prader-Willi Syndrome, Schaaf-Yang Syndrome, and Autism Spectrum Disorder. Int J Mol Sci. 2024;25(4):2066.
- 13. Salles J, Eddiry S, Lacassagne E, et al. Patients with PWS and related syndromes display differentially methylated regions involved in neurodevelopmental and nutritional trajectory. Clin Epigenetics. 2021;13(1):159.
- 14. Alavanda C, Arslan Ateş E, Yavaş Abalı Z, et al. Two new cases with novel pathogenic variants reflecting the clinical diversity of Schaaf-Yang syndrome. Clin Genet. 2023;104(1):127-132.
- 15. Halloun R, Habib C, Ekhilevitch N, et al. Expanding the spectrum of endocrinopathies identified in Schaaf-Yang syndrome - A case report and review of the literature. Eur J Med Genet. 2021;64(8):104252.
- 16. Rodriguez AM, Schain K, Jayakar P, et al. Report of two cases of Schaaf-Yang syndrome: Same genotype and different phenotype. Clin Case Rep. 2023;11(8):e7753.
- 17. Mizuno S, Yokoyama K, Yokoyama A, et al. Longitudinal analysis of electroencephalography pattern changes in an infant with Schaaf-Yang syndrome and a novel mutation in melanoma antigen L2 (MAGEL2). Mol Genet Genomic Med. 2022;10(6):e1932.
- 18. Schubert T, Schaaf CP. MAGEL2 (patho-)physiology and Schaaf-Yang syndrome. Dev Med Child Neurol. 2025;67(1):35-48.
- 19. Christian P. Schaaf, Institute of Human Genetics, Heidelberg University, Im Neuenheimer Feld 366, D-69120 Heidelberg, Germany.

