Background: Trapeziometacarpal (TMC) osteoarthritis is a degenerative disease of the thumb’s first carpometacarpal joint. Although several treatments can relieve symptoms, none clearly promote cartilage repair or modify disease progression. Regenerative therapies using adipose tissue have recently emerged as a and possible cartilage restoration promissing treatment.
Methods: A case series of 45 patients (63 joints) treated at Image Regenerative Clinic between January 2018 and December 2022 was analyzed. Patients (aged 41–77 years) underwent ultrasound- guided intra-articular transplantation of microfragmented adipose tissue (MFAT, Lipogems®). Outcomes were assessed pre-treatment and at 6 and 12 months with the Disabilities of the Arm, Shoulder and Hand (DASH) score and Visual Analog Scale (VAS). Patient satisfaction was graded on a 4-point scale (1 = poor; 4 = excellent).
Results: At 6 months, satisfaction ratings were poor (9/45), fair (31/45), good (4/45), and excellent (1/45). At 12 months, ratings improved to poor (8/45), fair (12/45), good (18/45), and excellent (7/45). VAS scores for the most symptomatic joint fell from 5.3 to 2.2 (Δ 3.1 points). Thirty-four of 45 patients showed ≥ 30-point DASH improvement. No patient required surgery within 12 months; eight patients with poor outcomes had surgery in the following year.
Discussion: the best results were observed in Eaton stages II and III, where joint structures still retain regenerative potential. In more advanced stage IV cases, clinical improvement was often temporary, although many patients still reported meaningful pain relief and functional recovery for several months—making MFAT a valuable bridge therapy that can postpone or reduce the need for surgery.
Conclusions: MFAT transplantation was safe, minimally invasive, and clinically effective in most patients, representing a valuable early or intermediate option for TMC osteoarthritis.
Keywords: Adipose derived stem cells, Rhizarthrosis, Regenerative medicine, Lipogems, Mfat, Trapeziometacarpal osteoarthritis
Trapeziometacarpal osteoarthritis (TMC OA), also known as rhizarthrosis, is one of the most frequent degenerative conditions affecting the hand. It is characterized by progressive cartilage loss in the first carpometacarpal joint, synovial membrane alterations, and subchondral bone remodeling, ultimately leading to chronic pain and loss of thumb function. This disorder predominantly affects women between the fourth and fifth decades of life and represents a major cause of functional disability, limiting basic daily tasks such as grasping, pinching, and fine manipulation.1-4
The etiologic factors include the anatomic morphology of the joint, ligament laxity, repetitive microtrauma, and hormonal influences. Diagnosis relies on clinical examination and imaging.5-8 From a diagnostic perspective, pain is typically localized at the base of the thumb and exacerbated by movements of opposition or pinch. Clinical maneuvers such as the Grind and Distraction tests are commonly used to confirm the diagnosis3,7 while radiographic imaging allows classification according to the Eaton staging system, which divides the disease into four progressive grades.1,3,9,10
In early stages, conservative management—manual therapy, splinting, and physical modalities-is the first line of treatment.11-16 Intra-articular corticosteroids or hyaluronic acid injections may offer temporary symptom relief but are often associated with tissue atrophy or degenerative side effects.17 Other alternative conservative therapies are intra-articular injection of platelet-rich plasma (PRP)18-25 and ozone.26,27 If conservative treatments fail, surgery such as trapeziectomy or arthroplasty is indicated;28-37 however, these procedures require long rehabilitation and carry risk of instability. Over the past decade, the focus of the scientific community has gradually shifted toward regenerative approaches, aimed not only at alleviating symptoms but also at restoring the biological function and homeostasis of the joint environment.
Neither traditional therapy nor surgery induces cartilage regeneration.20 Hence, regenerative strategies using adipose tissue-rich in mesenchymal stem cells (MSCs)—are gaining attention.38-40 This study evaluates ultrasound-guided microfragmented adipose tissue (MFAT) transplantation as a minimally invasive treatment for TMC OA.40-47
The Lipogems® System
Lipogems® is a closed, saline-filled system that gently microfragments fat tissue to ≈ 300 µm clusters, removing oil and debris while preserving the stromal vascular niche.48-51 The resulting viable graft acts as a natural bioreactor rich in pericytes and growth factors, supporting angiogenesis and tissue homeostasis. In orthopedic use, Lipogems has shown pain relief and potential cartilage regeneration on MRI.52-55
The purpose of the present study is to evaluate the clinical outcomes and safety profile of ultrasound-guided intra-articular injection of MFAT obtained with the Lipogems® system in patients affected by trapeziometacarpal osteoarthritis, treated at Image Regenerative Clinic (Milan, Italy) between 2018 and 2022.
Study population
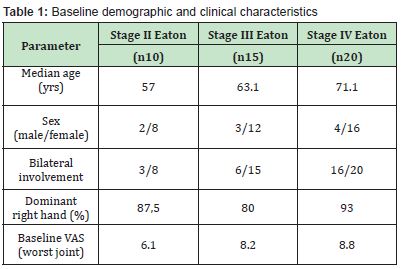
A retrospective case series was conducted on 45 consecutive patients (36 women, 9 men; aged 41–77 years) treated at Image Regenerative Clinic, Milan, between January 2018 and December 2022. Bilateral rhizarthrosis was observed in 25 patients, accounting for a total of 63 treated joints. Eaton staging distribution was: 10 stage II, 15 stage III, and 20 stage IV cases. Exclusion criteria included any previous hand surgery or intra-articular injection (corticosteroids, platelet-rich plasma, ozone) performed within 12 months before treatment, to avoid bias in outcome evaluation.
Surgical and injection procedure
Fat harvesting was performed under local anesthesia with light sedation using Klein’s solution. A volume ranging between 10 and 60 cc of lipoaspirate was processed through the Lipogems® closed, saline-filled device.
Under continuous ultrasound guidance, the processed MFAT was injected into the TMC joint using a 22-gauge needle, while applying axial traction to the thumb to optimize intra-articular distribution. The mean injected volume per joint was about 1 cc (range 0.5–2 cc). No postoperative immobilization was required. Patients were instructed to avoid heavy loads or pinch movements for four weeks.
A personalized rehabilitation protocol was initiated two weeks after the injection. This included neurodynamic mobilization and manual therapy techniques (Kaltenborn, Mulligan) and daily active exercises (~10 minutes per day). Two osteopathic sessions per month were prescribed for six months to promote functional recovery and improve joint alignment.
Outcome measures
Clinical assessment included: (a) pain intensity, measured with the Visual Analog Scale (VAS); (b) upper-limb function, evaluated by the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire; and (c) patient satisfaction, graded on a 4-point Likert scale (1 = poor, 4 = excellent). All scores were collected at baseline, 6 months, and 12 months after treatment. For bilateral cases, the most symptomatic joint was used for statistical analysis.
Overall outcomes
At the 6-month follow-up, satisfaction ratings were distributed as follows: poor = 9, fair = 31, good = 4, excellent = 1. After 12 months, results improved substantially: poor = 8, fair = 12, good = 18, excellent = 7. The mean VAS score for the most symptomatic joint decreased from 5.3 to 2.2 (Δ = 3.1 points). Thirty-four out of 45 patients (75.6%) achieved at least a 30-point improvement in their DASH score. None of the participants required surgery within the first year, while eight patients with persistently poor outcomes underwent surgical treatment during the following year. Clinical improvement correlated with Eaton stage but not with age Table 1.
Outcomes by disease stage
Stage II: Seven of ten patients (70%) reported excellent results, two were good, and one fair. Average VAS reduction was 4.3 points, with a mean DASH improvement of 25 points Table 2.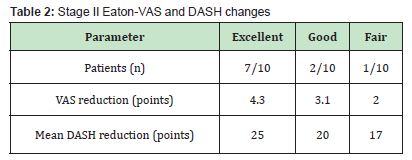
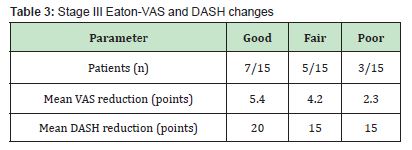
Stage III: Seven patients achieved good outcomes, five fair, and three poor. Mean VAS reduction reached 5.4 points among good responders, accompanied by an average 20-point improvement in DASH scores Table 3.
Stage IV: Ten patients reported good improvement, five fair, and five poor. Although the mean VAS drop was 5.3 points in the best responders, benefits tended to diminish over time, suggesting a transient effect in advanced disease stages Table 4.
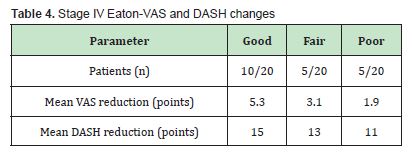
Eight patients later underwent trapeziectomy with arthroplasty, while nine opted to repeat the Lipogems® treatment.
Adherence and complications
Follow-up adherence was excellent, with all patients completing both 6- and 12-month evaluations. Rehabilitation compliance averaged ~70%. Only minor adverse events were observed: two small hematomas (resolved ≤14 days) and one case of transient superficial radial nerve paresthesia (resolved in 40 days). No infections, inflammatory reactions, or major complications were reported, confirming the high safety profile of the MFAT procedure.
Ultrasound-guided injection of microfragmented adipose tissue (MFAT) proved to be a safe, well-tolerated, and effective minimally invasive treatment for trapeziometacarpal osteoarthritis. The best results were observed in Eaton stages II and III, where joint structures still retain regenerative potential. In more advanced stage IV cases, clinical improvement was often temporary, although many patients still reported meaningful pain relief and functional recovery for several months—making MFAT a valuable bridge therapy that can postpone or reduce the need for surgery.
The therapeutic mechanism of MFAT relies on its ability to deliver a wide array of bioactive molecules within the joint microenvironment. Through preservation of the stromal vascular niche, the Lipogems® process maintains pericytes and mesenchymal progenitors capable of releasing cytokines, growth factors, and anti-inflammatory mediators.39,51 These components exert paracrine effects that modulate inflammation, enhance angiogenesis, and support cartilage and synovial tissue homeostasis. The micro-clusters (~300 µm) generated by the Lipogems® device integrate well into the local tissue, acting as a living “bioreactor” that continuously promotes cellular communication and repair.52
Rehabilitation played a crucial complementary role: patients who adhered consistently to the physiotherapy and osteopathic program achieved better and longer-lasting outcomes, underlining the importance of combining regenerative and functional therapies. The study’s limitations include its retrospective design, single-center setting, modest sample size, and absence of radiologic follow-up or control group. Nonetheless, the consistency of clinical improvement across stages II and III supports the potential of MFAT as an early or intermediate-stage therapy. Future randomized prospective trials with standardized protocols and MRI assessment are warranted to objectively evaluate cartilage regeneration and to define optimal patient selection criteria.
Lipogems® MFAT offers a biologically active, safe, and minimally invasive alternative for patients with early-to-moderate trapeziometacarpal osteoarthritis. It effectively reduces pain, improves hand function, and may delay surgical intervention, representing an important step toward the integration of regenerative medicine into everyday orthopedic and hand-surgery practice.
None.
This Research Article received no external funding.
Regarding the publication of this article, the authors declare that they have no conflicts of interest.
- 1. Gillis J, Calder K, Williams J. Review of thumb carpometacarpal arthritis classification, treatment and outcomes. Can J Plast Surg. 2011;19(4):134-138.
- 2. Saadeh PB, Kazanowski MA, Sharma S, et al. Rebalancing of forces as an adjunct to resection suspension arthroplasty for trapezial osteoarthritis. Ann Plast Surg. 2004;52(6):567-570.
- 3. Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J Hand Surg Br. 1994;19(3):340-341.
- 4. Herold C, Fleischer O, Allert S. Eigenfettinjektion in das Sattelgelenk zur Behandlung der Rhizarthrose - eine viel versprechende Therapieoption [Autologous fat injection for treatment of carpometacarpal joint osteoarthritis of the thumb - a promising alternative]. Handchir Mikrochir Plast Chir. 2014;46(2):108-112.
- 5. Brunelli GA, Monini L. Instability of the trapezio metacarpal joint: related arthritis and surgery. Surg Technol Int. 2000;9:252-258.
- 6. Pelligrini VD Jr. Osteoarthritis of the trapeziometacarpal joint: the pathophysiology of articular cartilage degeneration. II. Articular wear patterns in the osteoarthritic joint. J Hand Surg Am. 1991;16(6):975-982.
- 7. Pegoli L, Parolo C, Resnati E. Arthrosic pathology. In: Surgery and Rehabilitation of the Hand; Pajardi GE, Ed.; Piccin: Padua, Italy, 2014; Chapter 17.
- 8. Cook GS, Lalonde DH. MOC-PSSM CME article: Management of thumb carpometacarpal joint arthritis. Plast Reconstr Surg. 2008;121(1 Suppl):1-9.
- 9. Eaton RG, Lane LB, Littler JW, et al. Ligament reconstruction for the painful thumb carpometacarpal joint: a long-term assessment. J Hand Surg Am. 1984;9(5):692-699.
- 10. Eaton RG, Glickel SZ. Trapeziometacarpal osteoarthritis. Staging as a rationale for treatment. Hand Clin. 1987;3(4):455-471.
- 11. MacDermid JC, Wessel J, Humphrey R, et al. Validity of self-report measures of pain and disability for persons who have undergone arthroplasty for osteoarthritis of the carpometacarpal joint of the hand. Osteoarthritis Cartilage. 2007;15(5):524-530.
- 12. Chung KC, Pillsbury MS, Walters MR, et al. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg Am. 1998;23(4):575-587.
- 13. Winter R, Hasiba Pappas SK, Tuca AC, et al. Autologous Fat and Platelet-Rich Plasma Injections in Trapeziometacarpal Osteoarthritis: A Systematic Review and Meta-Analysis. Plast Reconstr Surg. 2023;151(1):119-131.
- 14. Villafañe JH, Cleland JA, Fernández de Las Peñas C. The effectiveness of a manual therapy and exercise protocol in patients with thumb carpometacarpal osteoarthritis: a randomized controlled trial. J Orthop Sports Phys Ther. 2013;43(4):204-213.
- 15. Colditz JC. The biomechanics of a thumb carpometacarpal immobilization splint: design and fitting. J Hand Ther. 2000;13(3):228-235.
- 16. Moulton MJ, Parentis MA, Kelly MJ, et al. Influence of metacarpophalangeal joint position on basal joint-loading in the thumb. J Bone Joint Surg Am. 2001;83(5):709-716.
- 17. Joshi R. Intraarticular corticosteroid injection for first carpometacarpal osteoarthritis. J Rheumatol. 2005;32(7):1305-1306.
- 18. Stahl S, Karsh Zafrir I, Ratzon N, et al. Corticosteroid vs. hyaluronic acid for degenerative trapeziometacarpal joints. J Clin Rheumatol. 2005;11:299-302.
- 19. Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15(Suppl 3):S2.
- 20. Hasiba Pappas S, Kamolz LP, Luze H, et al. Regenerative Therapies for Basal Thumb Arthritis-A Systematic Review. Int J Mol Sci. 2023;24(19):14909.
- 21. Pollard MA, Cermak MB, Buck WR, et al. Accuracy of injection into the basal joint of the thumb. Am J Orthop (Belle Mead NJ). 2007;36(4):204-206.
- 22. Froschauer SM, Holzbauer M, Wenny R, et al. Liparthroplasty for CMC OA: Two-year follow-up. J Clin Med. 2021;10:113.
- 23. Loibl M, Lang S, Dendl LM, et al. Leukocyte-Reduced Platelet-Rich Plasma Treatment of Basal Thumb Arthritis: A Pilot Study. Biomed Res Int. 2016;2016:9262909.
- 24. Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9(12):721-730.
- 25. Scotti C, Gobbi A, Karnatzikos G, et al. Cartilage Repair in the Inflamed Joint: Considerations for Biological Augmentation Toward Tissue Regeneration. Tissue Eng Part B Rev. 2016;22(2):149-159.
- 26. Latini E, Nusca SM, Curci ER, et al. Ozone and Hyaluronic Acid, Alone and in Combination: Exploring Temporal Dynamics and Synergies in Intraarticular Therapy for Knee Osteoarthritis. Pain Physician. 2025;28(4):347-357.
- 27. Rahimzadeh P, Imani F, Azad Ehyaei D, et al. Efficacy of Oxygen-Ozone Therapy and Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Meta-analysis and Systematic Review. Anesth Pain Med. 2022;12(4):e127121.
- 28. Berto GM, Pegoli L, Cortese PD, et al. Suspension arthroplasty in rhizarthrosis: 792 cases. Riv Chir Mano. 2010;47:1-8.
- 29. Burton RI, Pellegrini VD. Ligament reconstruction with tendon interposition arthroplasty. J Hand Surg Am. 1986;11:324-332.
- 30. Kriegs Au G, Petje G, Fojtl E, et al. Ligament reconstruction with or without tendon interposition to treat primary thumb carpometacarpal osteoarthritis. A prospective randomized study. J Bone Joint Surg Am. 2004;86(2):209-218.
- 31. Kleinman WB, Eckenrode JF. Tendon suspension sling arthroplasty for thumb trapeziometacarpal arthritis. J Hand Surg Am. 1991;16(6):983-991.
- 32. Pegoli L, Prashanth S, Calcagni M, et al. Suspension arthroplasty: Evaluation of 400 patients. J Hand Surg. 2005;10:199-203.
- 33. Mureau MA, Rademaker RP, Verhaar JA, et al. Tendon interposition arthroplasty versus arthrodesis for the treatment of trapeziometacarpal arthritis: a retrospective comparative follow-up study. J Hand Surg Am. 2001;26(5):869-876.
- 34. Forseth MJ, Stern PJ. Complications of trapeziometacarpal arthrodesis using plate and screw fixation. J Hand Surg Am. 2003;28(2):342-345.
- 35. Bohr S, Rennekampff HO, Pallua N. Cell-Enriched Lipoaspirate Arthroplasty: A Novel Approach to First Carpometacarpal Joint Arthritis. Hand Surg. 2015;20(3):479-481.
- 36. Raven EE, Kerkhoffs GM, Rutten S, et al. Long term results of surgical intervention for osteoarthritis of the trapeziometacarpal joint: comparison of resection arthroplasty, trapeziectomy with tendon interposition and trapezio-metacarpal arthrodesis. Int Orthop. 2007;31(4):547-554.
- 37. Makris EA, Gomoll AH, Malizos KN, et al. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21-34.
- 38. Ter Huurne M, Schelbergen R, Blattes R, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64(11):3604-3613.
- 39. Wu L, Cai X, Zhang S, et al. Regeneration of Articular Cartilage by Adipose Tissue Derived Mesenchymal Stem Cells: Perspectives From Stem Cell Biology and Molecular Medicine. J Cell Physiol. 2013;228:938-944.
- 40. Herold C, Rennekampff HO, Groddeck R, et al. Autologous Fat Transfer for Thumb Carpometacarpal Joint Osteoarthritis: A Prospective Study. Plast Reconstr Surg. 2017;140(2):327-335.
- 41. Tremolada C, Colombo V, Ventura C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr Stem Cell Rep. 2016;2(3):304-312.
- 42. Wilson EB. The Cell in Development and Inheritance; Macmillan: New York, NY, USA. 1898.
- 43. Canaider S, Maioli M, Facchin F, et al. Human stem cell exposure to zebrafish extracts. CellR4. 2014;2(5):1226.
- 44. Huang JI, Beanes SR, Zhu M, et al. Rat extramedullary adipose tissue as a source of osteochondrogenic progenitor cells. Plast Reconstr Surg. 2002;109(3):1033-1043.
- 45. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341-347.
- 46. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076-1084.
- 47. Lonardi R, Leone N, Gennai S, et al. Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: a randomized controlled single-center clinical trial (MiFrAADiF). Stem Cell Res Ther. 2019;10(1):223.
- 48. Bianchi F, Maioli M, Leonardi E, et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22(11):2063-2077.
- 49. Tassinari R, Canaider S, Pasquinelli G, et al. Lipogems, a New Modality of Fat Tissue Handling to Enhance Tissue Repair in Chronic Hind Limb Ischemia. CellR4. 2014, 2, e1289.
- 50. Ceserani V, Ferri A, Berenzi A, et al. Angiogenic and anti-inflammatory properties of micro-fragmented fat tissue and its derived mesenchymal stromal cells. Vasc Cell. 2016;8:3.
- 51. Tremolada C, Rocheteau P, Lavorano C, et al. Micro-fractured Adipose Tissue Graft (Lipogems), Regenerative Surgery and Potential Outcomes for Infectious and Cancer Diseases. J Stem Cell Res. 2023;4(2):53.
- 52. Bouglé A, Rocheteau P, Hivelin M, et al. Micro-fragmented fat injection reduces sepsis-induced acute inflammatory response in a mouse model. Br J Anaesth. 2018;121(6):1249-1259.
- 53. Mastrangelo AN, Haus BM, Vavken P, et al. Immature animals have higher cellular density in the healing anterior cruciate ligament than adolescent or adult animals. J Orthop Res. 2010;28(8):1100-1106.
- 54. Hudetz D, Borić I, Rod E, et al. The Effect of Intra-articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes (Basel). 2017;8(10):270.
- 55. Tremolada, C. Microfractured Adipose Tissue Graft (Lipogems) and Regenerative Surgery. J Orthop Res Ther. 2022;7:1210.
- 56. Guidi M, Marcovici LL, Ruas JS, et al. Autologous Fat Transfer for Finger Joint and Basal Thumb Osteoarthritis. In: Synovial Joints: New Research on Structure and Function; Intech Open. 2024.

